HHS is expanding its scientific understanding of how best to advance health care, public health, human services, biomedical research, and to ensure the availability of safe medical and food products. Chief among these efforts is the identification, implementation, and rigorous evaluation of new approaches in science, health care, public health, and human services that encourage efficiency, effectiveness, sustainability, and sharing or translating that knowledge into better products and services.
Whole Genome Sequencing
CDC now has almost two years of data on the impact of Advanced Molecular Detection (AMD) technologies (specifically, whole genome sequencing, or WGS) on foodborne listeriosis. Since the adoption of WGS, the number of outbreaks detected has increased by 50% while the number of cases per outbreak has decreased by 50%. Outbreaks are being detected earlier and the number of cases linked to specific food sources has increased 15-fold.
Goal Two includes four objectives:
- Accelerate the process of scientific discovery to improve health
- Foster and apply innovative solutions to health, public health, and human services challenges
- Advance the regulatory sciences to enhance food safety, improve medical product development, and support tobacco regulations
- Increase our understanding of what works in public health and human service practice
- Improve laboratory, surveillance, and epidemiology capacity
About one-third of breast cancer patients eventually develop metastases, or tumors formed by cancer cells that have spread to other organs. Screening is an important aspect of follow-up care for breast cancer patients, and early detection of recurrence is critical in tailoring appropriate and effective treatments. The earliest signs of cancer spread are called micrometastases, which are often too small to be detected with standard screening. However, there is a promising development based on recent NIH-funded research. Using a biochemical approach researchers developed a probe that combined with magnetic resonance imaging (MRI) successfully detected very tiny tumors of just a few hundred cells, or as small as one-hundredth of an inch, in mice. This technique also has the potential to differentiate aggressive tumors from low-risk tumors. The researchers are conducting additional work with the goal of testing this technique in human trials. If successful, this technique may offer an improved way to detect early metastatic spread, which would allow adaptation of treatment more quickly and lead to better outcomes for patients.
FDA microbiologists are evaluating and integrating commercially available instrumentation into its microbiological testing workflow that is vastly improving the ability of FDA to more quickly and effectively detect and characterize foodborne pathogens such as Salmonella directly from the food supply. Improvements in sample throughput, along with the high degree of sensitivity and specificity built into new pathogen detection technologies, will dramatically improve FDA’s foodborne response and traceback capabilities. When fully deployed, technologies such as next-generation whole-genome sequencing (WGS) and others will reduce the time to conduct these analyses from 14 days originally to just a few days. One updated technology which provides highly accurate and rapid Salmonella serotype results for FDA, known as the flow cytometry/fluorescence platform, has been validated extensively and is now deployed in nearly all FDA field laboratories, as well as in CFSAN and CVM laboratories. In FY 2015, FDA exceeded the target of four working days, reducing the average number of days to serotype priority pathogens in foods to three working days, which is the minimum amount of time required. The proposed target for FY 2016 and FY 2017 is three working days, which will maintain the level achieved in FY 2015.
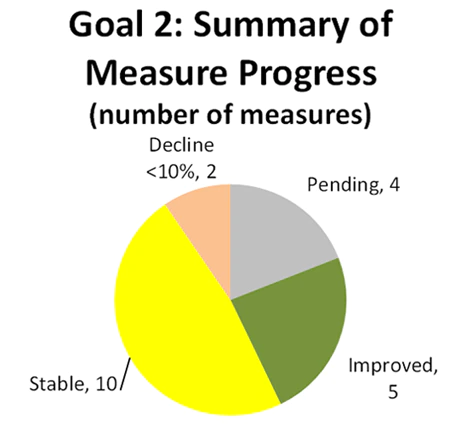
For this goal, 88 percent of measures with available data showed stable or improved performance.