COVID-19 Diagnostic Data Standards: Frequently Asked Questions
To support a data-driven U.S. response to the coronavirus pandemic, public health authorities and HHS depend on key data elements reported efficiently and accurately using COVID-19 data standards to inform action. The following existing guidance and technical specifications direct adoption for real-world impact.
- HHS Guidance: COVID-19 Pandemic Response, Laboratory Data Reporting: CARES Act Section 18115 (June 4, 2020; updated January 8, 2021)
- COVID-19 Data Reporting for Laboratory-Based Testing (August 31, 2020) (Technical Specifications for Implementation)
- COVID-19 Data Reporting for Non-Laboratory-Based Testing (September 23, 2020) (Technical Specifications for Implementation)
What is diagnostic data?
In general, diagnostic data refers to information collected and used in the investigation and diagnosis of a disease. For the COVID-19 pandemic, U.S. diagnostic data refers to information collected from laboratory and non-laboratory diagnostic testing for the purposes of clinical diagnosis and public health screening.
Can HHS provide additional information, including technical specifications, to support laboratories with implementation?
Yes, there are detailed, technical specifications, examples, and links to underlying coding elements have been provided to support the clinical and laboratory stakeholder communities in adopting and implementing consistent and harmonized data capture, coding, and reporting. Please see the following resources for further details:
- COVID-19 Data Reporting for Laboratory-Based Testing (August 31, 2020) (Technical Specifications for Implementation)
- COVID-19 Data Reporting for Non-Laboratory-Based Testing (September 23, 2020) (Technical Specifications for Implementation)
- HHS ELR Submission Guidance using HL7 v2 Messages
- HHS Guidance: COVID-19 Pandemic Response, Laboratory Data Reporting: CARES Act Section 18115 (June 4, 2020; updated January 8, 2021)
- Prior HHS answer to this question (July 2020).
As described in the guidance, anyone who orders a COVID-19 test, collects a specimen, or performs a laboratory test should make every reasonable effort to collect complete demographic information as well as data for "ask on order entry" (AOE) questions. To facilitate this in the clinical community, CDC has provided further information for healthcare providers and additional information at How to Report COVID-19 Laboratory Data.
What diagnostic data elements should be captured and reported from COVID-19 diagnostic tests?
The CARES Act required specified data elements must be reported for COVID-19 diagnostic tests, and these data elements were further defined through HHS Guidance: COVID-19 Pandemic Response, Laboratory Data Reporting: CARES Act Section 18115 (June 4, 2020; updated January 8, 2021) and COVID-19 Data Reporting for Laboratory-Based Testing (August 31, 2020) (Technical Specifications for Implementation) and COVID-19 Data Reporting for Non-Laboratory-Based Testing (September 23, 2020) (Technical Specifications for Implementation) issued by HHS. The mandatory reporting requirement was needed to ensure that critical laboratory-based diagnostic testing data is being collected and reported to state and federal public health officials to inform the pandemic response at every level across the United States.
Stakeholders have asked for clarification as to which data elements should be prioritized for collection and reporting and which data elements should be collected at a minimum for state and federal reporting. Therefore, HHS is providing the "Mandatory Minimum Core Data Elements for All COVID-19 Diagnostic Test Reporting" table. This table, now available as an Excel download, represents the data elements identified in the CARES Act and defined in the HHS COVID-19 technical specifications for laboratory-based and non-laboratory-based tests combined into one streamlined table, and it also clarifies which data elements are considered the mandatory minimum for federal and state capture and reporting of laboratory-based testing at this time.
The COVID-19 diagnostic technology landscape is evolving rapidly. Non-laboratory-based tests (those performed outside of a CLIA-certified location, either by prescription or non-prescription and over-the-counter), should have the capability of capturing all mandatory and requested data elements for both state and federal level reporting. Device developers and manufactures, as well as digital health and software developers, could facilitate this for non-laboratory-based testing devices. Federal data reporting interfaces—for example, the Wireless Automated Transmission for Electronic Reporting Systems (WATERS), COVID-19 electronic laboratory reporting (CELR), or SimpleReport—are capable of receiving all mandatory and requested core federal data elements from all non-laboratory-based diagnostic reporting systems. State level reporting capabilities (such as the ability of states to receive all core data from non-laboratory-based tests) may not yet exist in every jurisdiction. As the reporting pathways and capabilities for data receipt are further refined for state-level reporting, it is encouraged that all data capture capabilities and diagnostic workflows be built in accordance with the table at this time, and further information will be provided as it becomes available. HHS, in consultation with the Council for State and Territorial Epidemiologists (CSTE) and the Association of Public Health Laboratories (APHL), strongly encourages diagnostic test and device manufacturers to enable data collection and reporting of test results to support disease (or condition) reporting per COVID-19 public health response and state reporting data needs and requirements for tests performed in all settings including point of care and at home testing.
Is there a machine-readable version of the core data elements for COVID-19 diagnostic tests?
Yes, information in the below table is now available in .csv file format.
TABLE: Mandatory Minimum Core Data Elements for All COVID-19 Diagnostic Test Reporting
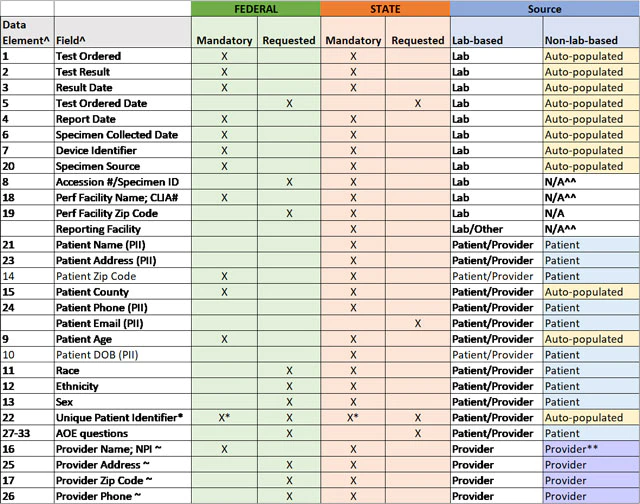
^ per COVID-19 Data Reporting for Laboratory-Based Testing (August 31, 2020) (Technical Specifications for Implementation) and COVID-19 Data Reporting for Non-Laboratory-Based Testing (September 23, 2020) (Technical Specifications for Implementation)
* Mandatory for non-laboratory-based tests only
~ if by prescription; n/a for non-prescription tests
^^ enter "SA" for self-administered in these fields
** enter "OTC" for over-the-counter in these fields, if non-prescription
Where can I find additional resources and information on COVID-19 diagnostic data standards?
- HHS Announces New Laboratory Data Reporting Guidance for COVID-19 Testing
- HHS Guidance: COVID-19 Pandemic Response, Laboratory Data Reporting: CARES Act Section 18115 (June 4, 2020; updated January 8, 2021)
- COVID-19 Data Reporting for Laboratory-Based Testing (August 31, 2020) (Technical Specifications for Implementation)
- COVID-19 Data Reporting for Non-Laboratory-Based Testing (September 23, 2020) (Technical Specifications for Implementation)
- HHS ELR Submission Guidance using HL7 v2 Messages
- How to Report COVID-19 Laboratory Data
Have other questions or want assistance? Please send questions, comments, or requests for technical support to: DLSinquiries@cdc.gov

