The Hepatitis C Medicaid Affinity Group (Affinity Group) aims to increase the number and percentage of Medicaid beneficiaries diagnosed with hepatitis C virus (HCV) who are successfully treated and cured.
It provides support to states to develop and implement their own initiatives, provide information and technical assistance, and explore strategies related to HCV in specific settings, such as correctional and behavioral health.
Many of the resources, state strategies, and other information is available through the links below.
Participating States
The efforts of the Affinity Group are driven by state teams made up of representatives from state Medicaid agencies, public health departments, correctional agencies, and other programs, such as those that address behavioral health care and substance use disorders.
All states were invited to join. Nine states participate in its third year (2020); five are returning states. Nineteen jurisdictions in total have participated in the Affinity Group, including Los Angeles County and Washington, DC.
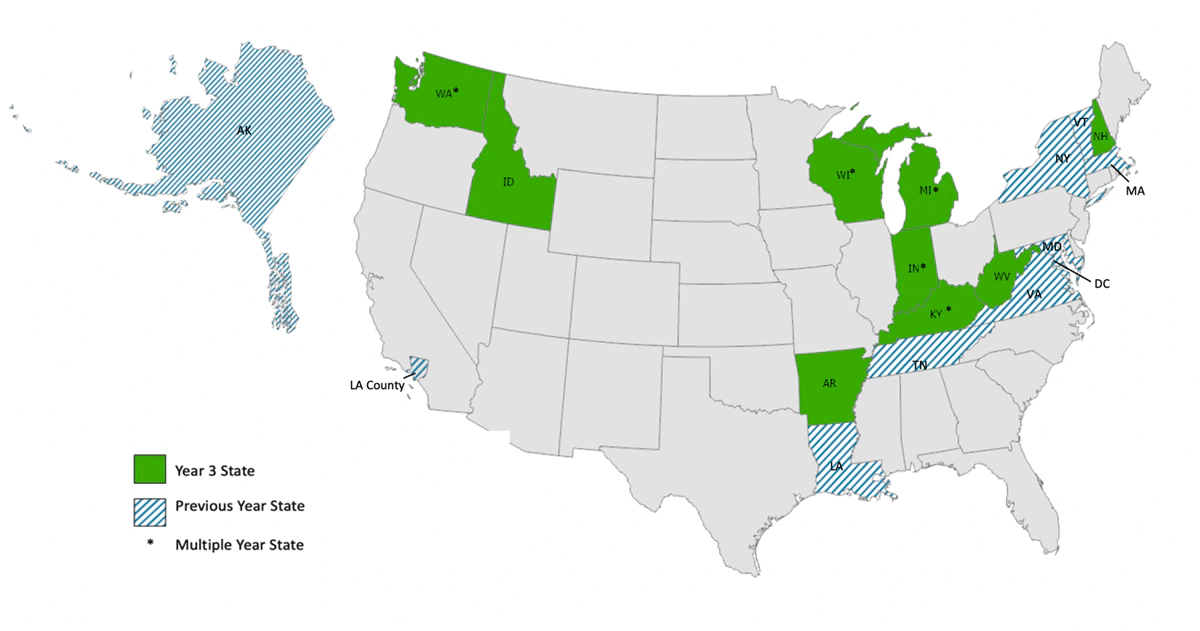
Year 3 State Participants: Arkansas, Idaho, *Indiana, *Kentucky, *Michigan, New Hampshire, *Washington, West Virginia, and *Wisconsin
Previous Year State Participants: Alaska, District of Columbia, Los Angeles County, Louisiana, Maryland, Massachusetts, New York State, Tennessee, Vermont, and Virginia
New HCV treatments can result in a cure for approximately 95% of people who take them. People who are cured of HCV experience multiple health benefits and are significantly less likely to develop severe liver disease, liver cancer, and liver failure, which are often very costly conditions. Eliminating hepatitis requires increasing access to screening, diagnosis, and early HCV treatment, which together will save lives, reduce new infections, and control health care costs. These goals are described in the The Viral Hepatitis National Strategic Plan: A Roadmap to Elimination 2021-2025.
States are key leaders in public health innovation, but do not always have opportunities to share effective strategies and collaborate to identify solutions to common challenges. The Affinity Group addresses this need by bringing states together to support developing and implementing innovative strategies for scaling up HCV screening, treatment, and cure.
The Affinity Group is a continuous quality improvement project that supports the development of evidence-based practices and implementation strategies to improve Medicaid systems’ efforts to address HCV. The project aims to:
- Foster state collaboration and share promising practices;
- Develop and implement innovative HCV-related policies and programs;
- Increase the number and percentage of Medicaid beneficiaries diagnosed and successfully treated (or cured) for HCV.
Convened by the Office of Infectious Disease and HIV/AIDS Policy (OIDP) in the Office of the Assistant Secretary for Health (OASH), the Affinity Group is a collaborative effort across the Department of Health and Human Services (HHS). It also engages Centers for Medicare & Medicaid Services (CMS), Centers for Disease Control and Prevention (CDC), Health Resources and Services Administration (HRSA), and Substance Abuse and Mental Health Services Administration (SAMHSA) to provide technical assistance and consultation to participating states.
Examples of Affinity Group activities undertaken by states:
- Using data to inform policy decisions: States have developed data use agreements between state Medicaid programs, public health agencies, and corrections departments to share data on HCV screening, diagnosis, and treatment. By calculating care cascades, states aim to identify screening and treatment patterns and develop targeted approaches to improve access. Populations of interest include individuals co-infected with HIV, people who inject drugs, women, and infants born to women with HCV.
- Making direct-acting antivirals (DAAs) more affordable: States are addressing the high cost of HCV treatment by developing innovative payment arrangements including subscription payment models and 340B pricing for covered entities.
- Removing structural barriers to DAAs: Multiple states have removed fibrosis score restrictions, prescriber specialty requirements, and restrictions related to substance use. States are also assessing the financial and clinical impact of streamlining or removing Medicaid prior authorization requirements.
- Enhancing provider capacity for HCV testing and treatment: States have developed new curricula, trained providers, and implemented pharmacist-led treatment models. States are also increasing provider capacity through Project ECHO, a tele-mentoring program. In addition, they are analyzing data to identify high-burden areas and prescriber patterns to inform provider capacity-building efforts.
- Increasing treatment access for people who inject drugs and in correctional settings: States are exploring opportunities to co-locate HCV screening and treatment with treatment for substance use disorder. In addition, several participants have proposed increasing screening and treatment rates of inmates and improving care coordination efforts so individuals can enroll into Medicaid and continue or start HCV treatment upon release from incarceration.
The Affinity Group prepares technical assistance documents and resources to assist states in implementing their HCV strategies. In addition, the Affinity Group conducts an evaluation at the end of each year to assess whether (1) how well states achieved their goals, (2) the strength of states’ reported engagement and satisfaction with Affinity Group activities, (3) how well the Affinity Group helped advance state activities and improve collaboration across state entities, and (4) ways to improve the Affinity Group and other similar initiatives. Resources developed under the Affinity Group include:
Technical Assistance Documents
- Medicaid Provider Training for Hepatitis C Virus. Summary of available provider training resources and state-specific examples of successful provider training initiatives
- Identifying People Who Inject Drugs (PWID) in Medicaid Claims. Overview of how to identify PWID in Medicaid claims data to support reporting of Affinity Group outcomes measures
- HCV Medicaid Drug Pricing. Overview of the Medicaid Drug Rebate Program, Medicaid Supplemental Rebates, and other potential strategies for states to manage costs associated with outpatient prescriptions
Evaluations
- Hepatitis C Medicaid Affinity Group: Evaluation Summary 2018-2019. Evaluation summary outlining key findings from the Year 1 and Year 2 Affinity Group evaluations, including state progress on goals, state engagement and satisfaction with the Affinity Group, collaboration successes and challenges, calculation of HCV outcomes measures, lessons learned, and considerations for future Affinity Groups
Case Studies
- Tackling Hepatitis C in Wisconsin. Case study outlining strengths, challenges, and opportunities related to Wisconsin’s strategies for addressing HCV. Wisconsin has participated in three years of the Affinity Group
- Eliminating Hepatitis C in Louisiana: An Innovative Payment and Outreach Model Study. Case study highlighting Louisiana’s modified subscription payment model, which aims to dramatically improve access to a selected DAA for Medicaid beneficiaries and incarcerated patients with HCV. Louisiana participated in Year 1 of the Affinity Group
HCV Guidelines
- HCV Guidance: Recommendation for Testing, Managing, and Treating Hepatitis C – AASLD and IDSA
- Hepatitis C Virus Infection in Adolescents and Adults: Screening – USPSTF
Resources for Providers
- Hepatitis C Prescriber Toolkit – HRSA
- Project ECHO – University of New Mexico School of Medicine
- Hepatitis C Online – Created by the University of Washington; funded by the CDC
- HIV/HCV Co-Infection: An AETC National Curriculum – Created by Rutgers School of Nursing; supported by HRSA
- Addiction Technology Transfer Centers (ATTCs) – Funded by SAMHSA
- Universal Hepatitis C Virus (HCV) Screening and Treatment Programs in Community Health Centers (webinar series) – National Nurse-Led Care Consortium
Data Tools
- Hep C State Policy Simulator – Created by Massachusetts General Hospital and Harvard Medical School; Funded by the CDC
- Hepatitis C: State of Medicaid Access – National Viral Hepatitis Roundtable (NVHR) and Center for Health Law and Policy Innovation, Harvard Law School
- Hep C Calculator – Created by Rollins School of Public Health at Emory University, Massachusetts General Hospital and Harvard Medical School; supported by the World Health Organization and UNITAID
- HepCorrections – Created by Massachusetts General Hospital and Harvard Medical School; funded by the National Science Foundation
- MappingHepC.com – AbbVie Inc.
Payment Models
- MassHealth Value-Based Pharmacy Purchasing Proposal – MassHealth
- Request for Information on Subscription Payment Models – Louisiana Department of Health
- Novel State Payment Models for Prescription Drugs: Early Implementation Successes and Challenges Created by Margolis Center for Health Policy; funded by the Robert Wood Johnson Foundation
Other Resources for State Agencies
- Case Studies: Medicaid Managed Care Plan Best Practices in Hepatitis C Linkage to Care, Treatment, and Retention – NASTAD
- State Strategies for Establishing Connections to Health Care for Justice-Involved Populations: The Central Role of Medicaid – The Commonwealth Fund
- Health Home Information Resource Center – CMS
Affinity Group state participants met with federal partners and other subject matter experts in Washington DC on February 8-9, 2018 for the first in-person convening of the Affinity Group. The in-person convening helped states obtain and share information to advance their goal of increasing the number of people cured of HCV. State participants also meet with subject matter experts during monthly calls to continue their efforts throughout the year. Links to some of the presentations are below.
General HCV
- What’s New in HCV at the CDC? (2020). CDC strategic priorities and key policy and program changes supporting those priorities, including new HCV screening guidelines and a combined surveillance and prevention funding opportunity. Carolyn Wester (MD, MPH), CDC
- Updates from Non-Governmental Partners in Combatting HCV (2020). HCV-related TA efforts of key non-governmental allies. Boatemaa Ntiri-Reid (JD, MPH), NASTAD, and Lauren Canary (MPH), National Viral Hepatitis Roundtable
- Eliminating Hepatitis C in New York State (2019). New York’s statewide HCV elimination strategy, including removing barriers to care, expanding access to care, establishing targets and metrics, and timeline for implementation. Colleen Flanigan (RN, MS), New York State Department of Health
- Technical Assistance Available through NASTAD (2019). Alyssa Kitlas, NASTAD
- Viral Hepatitis: We Can Eliminate Hepatitis C, Update on the Most Recent AASLD Recommendations. Bruce Luxon, MD, PhD from the American Association for the Study of Liver Diseases (AASLD) and Georgetown University presented the latest AASLD treatment guideline: “Treatment is recommended for all patients with chronic HCV infection, except those with short life expectancies that cannot be remediated by treating HCV...”. He also described the progression of liver disease with HCV, changes in cure rates with medication advances, and the cost effectiveness of treatment.
Using Data to Inform Policy Decisions
- Global Hepatitis Outbreak & Surveillance Technology (2019). How CDC’s GHOST technology can identify and address HCV hotspots. Yury Khudyakov (PhD), CDC
- Developing a Registry to Coordinate HCV Screening and Linkage to Care (2019). The development of a registry in the Los Angeles County Jail system to track the status of essential steps in the HCV care cascade, results needed to plan treatment, and information necessary for care coordination. Meredith Haddix (MPH), Los Angeles County Department of Public Health
- Data-to-Care Model Using Facility-Specific Hepatitis C Dashboards (2019).. How New York City has distributed data dashboards to acute-care hospitals and community health centers with two metrics (RNA confirmation rate and treatment initiation rate) to promote HCV testing and treatment. Miranda Moore and Nadine Kela-Murphy, New York City Department of Health and Mental Hygiene
- Tennessee, A New Dawn of Collaboration (2019). The growing collaboration between the Department of Health and the Department of Corrections, including opt-out HCV intake screening implemented November 2018. Kimberly Gill (MPH, BSN, RN), Tennessee Department of Health
- HCV Care Cascade, New York State Experience (2019). New York’s HCV Care and Treatment Initiative’s data reporting requirements, which assist the state in tracking treatment status. Colleen Flanigan (RN, MS), New York State Department of Health
- HCV Care Cascade, Wisconsin Experience (2019). Wisconsin’s development of a care cascade for women of childbearing age and next steps to further the state’s efforts. Ruth Koepke (MPH), Wisconsin Department of Health Services
- Machine Learning and Predictive Models in Hepatitis C (2019). Using machine learning to help target treatment of HCV. Akbar K. Waljee (MD, Msc), University of Michigan Health Systems and David Neff (DO), Michigan Department of Health and Human Services
- Pregnant Women & Infants: Improving the Hepatitis C Care Cascade (2019).. Testing and linkage to care recommendations for pregnant women and infants. Rachel Epstein (MD, MA), Boston Medical Center
- Assessment of Injection Drug Use through Administrative Data (2018). Methods for identifying people who inject drugs through administrative data, including testing, medical visits, prescriptions, and mortality data, to construct a cascade of care for HCV. Naveed Zafar Janjua (MBBS, MSc, DrPH), British Columbia Centre for Disease Control and the School of Population & Public Health, University of British Columbia
Making DAAs More Affordable
- Pharmacy Purchasing in Washington State (2019).. Implementation of Washington’s modified subscription payment model. Mary Fliss (MHA) and Donna Sullivan (PharmD, MS), Washington State Health Care Authority
- Assessing the Burden of Illness of Chronic Hepatitis C and Impact of Direct-Acting Antiviral Use on Healthcare Costs in Medicaid (2019).. Overview of a study using Medicaid claims data from 16 states that assessed burden of HCV illness and found that, on average, DAA costs are fully offset by benefits after only 14 months. M. Christopher Roebuck (PhD MBA), RxEconomics LLC
- Drug Pricing in Corrections (2019). Overview of policies regardingdiscounted drug pricing to correctional facilities and challenges related to limited state negotiating power. Sean Dickson (MPH, JD), The Pew Charitable Trusts
- Pharmaceuticals: State Strategies to Improve Access While Ensuring Fiscal Sustainability (2018). Drug pricing trends in specialty medicines and two strategies identified in the National Governors Association (NGA) 2018 report, Public Health Crisis and Pharmaceutical Interventions: Improving Access While Ensuring Fiscal Sustainability, the Medicaid spending cap for pharmaceuticals and the Subscription Model. Kate Johnson (MPH), NGA
- VA HCV Drug Price Negotiation Process (2018). The evolution of hepatitis C treatment in the VA and the impact of VA formulary management practices on hepatitis C medication pricing. Jennifer Zacher (PharmD), U.S. Department of Veteran Affairs
- Eliminating Hepatitis C in Louisiana: DAA Subscription Model (2018). Louisiana’s plan to eliminate HCV through a subscription payment model, i.e., paying a drug manufacturer for unlimited access to the treatment for individuals enrolled in Medicaid or in the correctional system for a set cost over a set contractual period. Alex Billioux (MD, DPhil), Louisiana Office of Public Health
Removing Structural Barriers to DAAs
- Managed Care and HCV Treatment Access (2019).. Importance of state monitoring of DAA restriction parity between Medicaid fee-for-service and managed care. Lauren Canary (MPH), National Viral Hepatitis Roundtable
- HCV Elimination by 2030 (2019). Barriers to HCV treatment in the U.S., progress made in removing restrictions from 2014 to 2019, and what can be done moving forward to achieve HCV elimination. Michael Ninburg (MPA), Hepatitis Education Project
- Liberalization of Coverage for DAAs in Wisconsin (2019). Policy changes made to remove DAA restrictions and post-policy utilization and fiscal impact in the state of Wisconsin. Julie Sager (MD), Wisconsin Division of Medicaid Services
- Prior Authorization of Hepatitis C Medications in NYS Medicaid Fee for Service (FFS) and Medicaid Managed Care. Monica Toohey, RPh from the New York State Department of Health described the state’s Drug Utilization Review process and timeline to first implement and then lift DAA reimbursement criteria. To streamline DAA prior authorization, the state relies on automation and encourages managed care organizations to use standardized criteria and processes.
- HCV Medication Prior Authorization (2018). Strategies for addressing Medicaid and other insurers’ prior authorization requirements for HCV medications and the role of pharmacist clinicians in HCV care. Paulina Deming (PharmD), University of New Mexico
Enhancing Provider Capacity for HCV Testing and Treatment
- Clinical Pharmacist Care Models for HCV Management (2018). The role of pharmacists in HCV management and how the HCV PharmD provider framework could be replicated in other settings. Pamela S. Belperio (PharmD, BCPS, AAHIVP), Department of Veterans Affairs
- Indiana Medicaid Partnership with Indiana University Project ECHO (2018).. Program to create access to high-quality specialty care. John Ross (RPh, RN), Indiana Family and Social Services Administration
- Project ECHO and HCV Treatment (2018). Medicaid financing models for Project ECHO and other potential financing mechanisms. Greg Howe, Center for Health Care Strategies
Targeting At-Risk Populations: Injection Drug Use
- American Correctional Association (ACA) Hepatitis C Initiatives (2019).. Changing landscape of treating HCV in correctional settings and related resources offered by ACA. Doreen Efeti (MPH, MBA, MCHES), ACA
- Hepatitis C Care in Los Angeles County Jails (2019).. Proposed workflow for HCV care of individuals in jails and the challenges to HCV treatment that jails face. Lauren Wolchok (MD), Los Angeles County Correctional Health Services
- Select States’ Reentry Processes from State Prison (2019).. Reentry processes from state prison in Arizona, Louisiana, and Ohio. Maria Schiff (MS), The Pew Charitable Trusts
Targeting At-Risk Populations: Injection Drug Use
- Reframing Reinfection: Public Health Strategies for the Era of Hepatitis C Elimination (2018). Importance of treating people with injection drug use for HCV despite risk of re-infection and strategies for preventing re-infections. Daniel Raymond, Harm Reduction Coalition
- HCV Testing and Treatment in the Corrections Context (2018). Barriers and opportunities to treating incarcerated populations for HCV. Mandy Altman, Hepatitis Education Project
- Treating People Who Inject Drugs (PWID) for HCV (2018). Benefits and facilitators of treating people who inject drugs with HCV. Lynn E. Taylor (MD, FACP, FAASLD), University of Rhode Island and Director of HIV and Viral Hepatitis Services, CODAC Behavioral Health

