Background:
Section 289 of the Public Health Service Act authorizes OHRP, on behalf of the U.S. Department of Health and Human Services (HHS), to establish a compliance oversight process regarding violations of the rights of human subjects of research conducted or supported by HHS. Pursuant to this authority, OHRP may receive reports of such violations and take appropriate action.
OHRP also derives compliance authority from the HHS regulations for the protection of human research subjects at 45 CFR part 46 (hereinafter referred to as "the HHS regulations"). HHS regulations at 45 CFR 46.103(a) require each institution engaged in non-exempt human subjects research that is conducted or supported by HHS to provide written assurance that it will comply with the requirements of the HHS regulations. On behalf of HHS, OHRP reviews and approves these written agreements, called assurances of compliance (Federalwide Assurances or FWAs). An FWA approved by OHRP commits the entire institution (including institutional officials, IRBs designated in the FWA, research investigators, and all other employees or agents) to full compliance with the HHS regulations whenever the institution is engaged in HHS-supported research.
Division of Compliance Oversight (DCO) Activity Data:
OHRP has responsibility for oversight of compliance with the Department of Health and Human Services (HHS) regulations for the protection of human research subjects (at 45 CFR Part 46). In carrying out this responsibility, DCO is the division within OHRP that reviews allegations of noncompliance these regulations, and determines whether to conduct a for-cause compliance investigation. DCO also conducts a human research protection program evaluations, and reviews incident reports (IRPTs) submitted by institutions that have a Federalwide Assurance on file at OHRP. These incidents include: reports of unanticipated problems involving risks to subjects or others; serious or continuing noncompliance with HHS regulations; and, suspensions or terminations of IRB approval.
The following tables and charts provide quarterly aggregate data per fiscal year (October 1st thru September 30th) on several key activities performed by DCO.
2021
| 2021 | 1Q Fiscal 2021 | 2Q Fiscal 2021 | 3Q Fiscal 2021 | 4Q Fiscal 2021 |
|---|---|---|---|---|
IRPTs |
||||
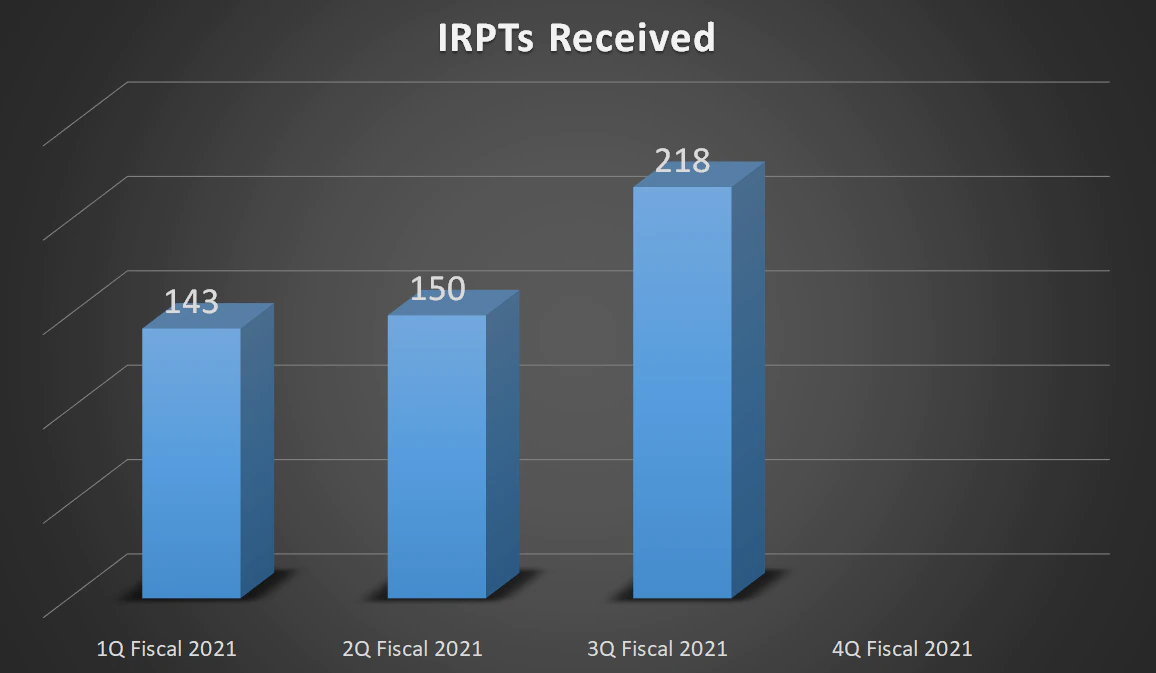
| Total IRPTs Received | 143 | 150 | 218 | - |
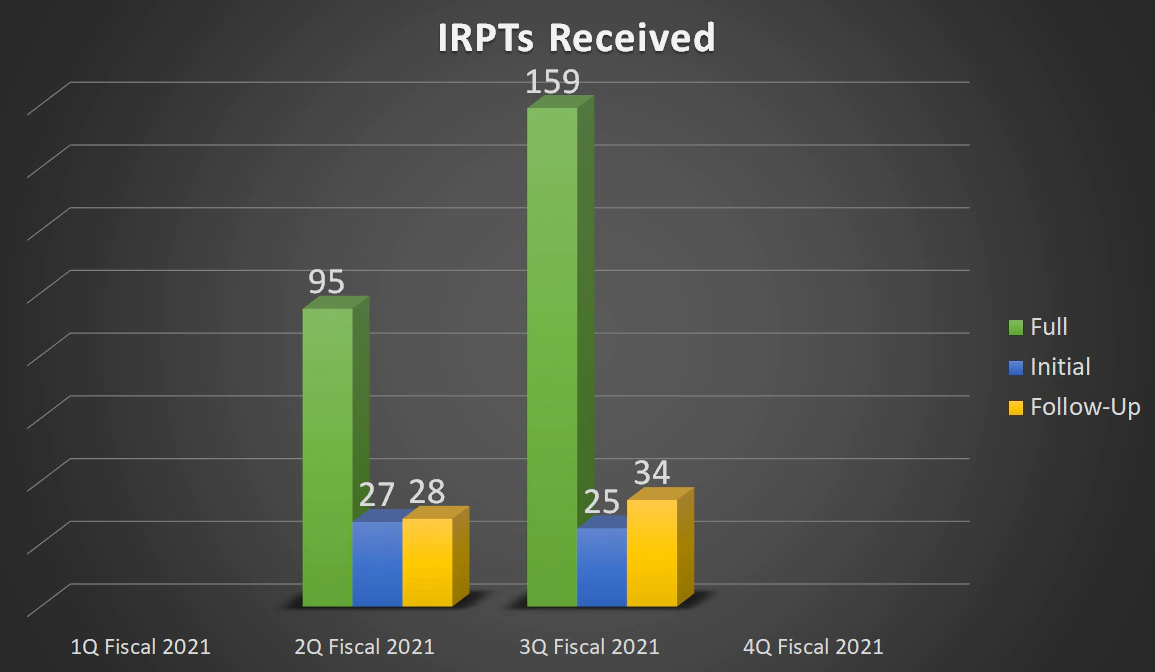
| Full | - | 95 | 159 | - |
| Initial | - | 27 | 25 | - |
| Follow-Up | - | 28 | 34 | - |
Complaints |
||||
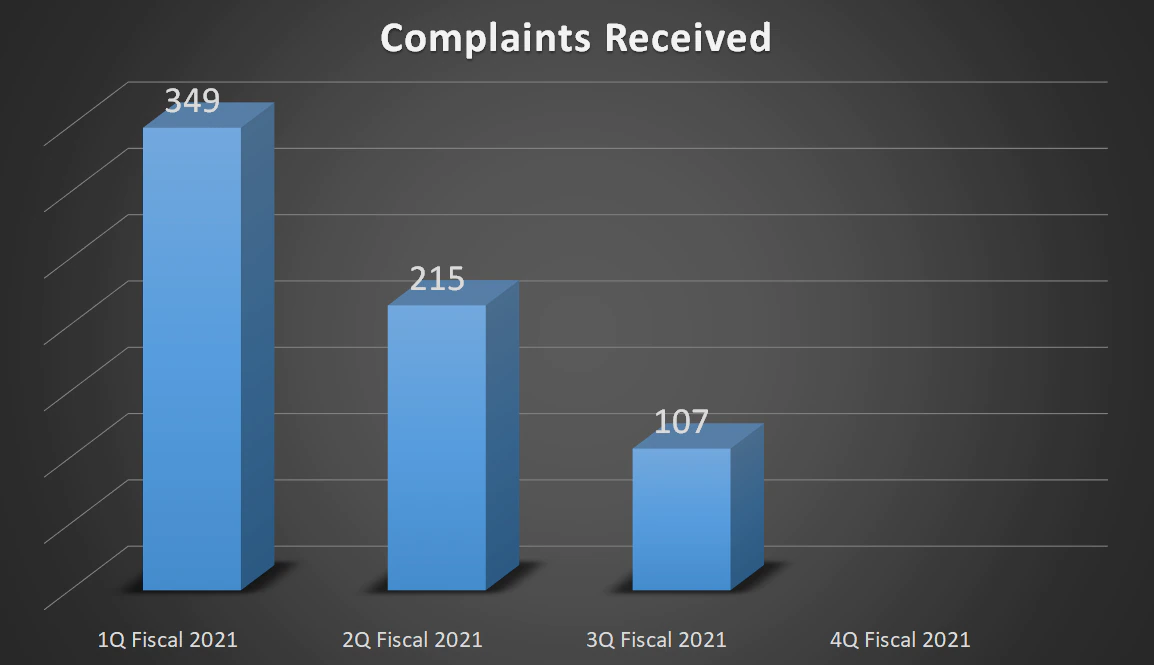
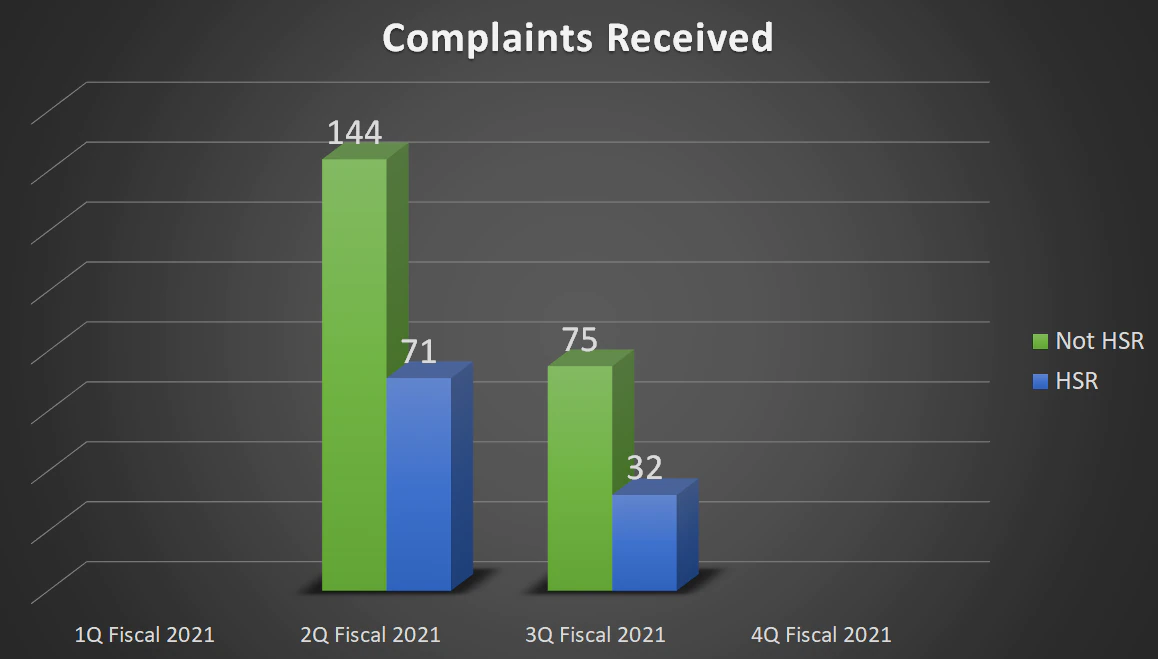
| Complaints Received | 349 | 215 | 107 | - |
| Not HSR | - | 144 | 75 | - |
| HSR | - | 71 | 32 | - |
Cases |
||||
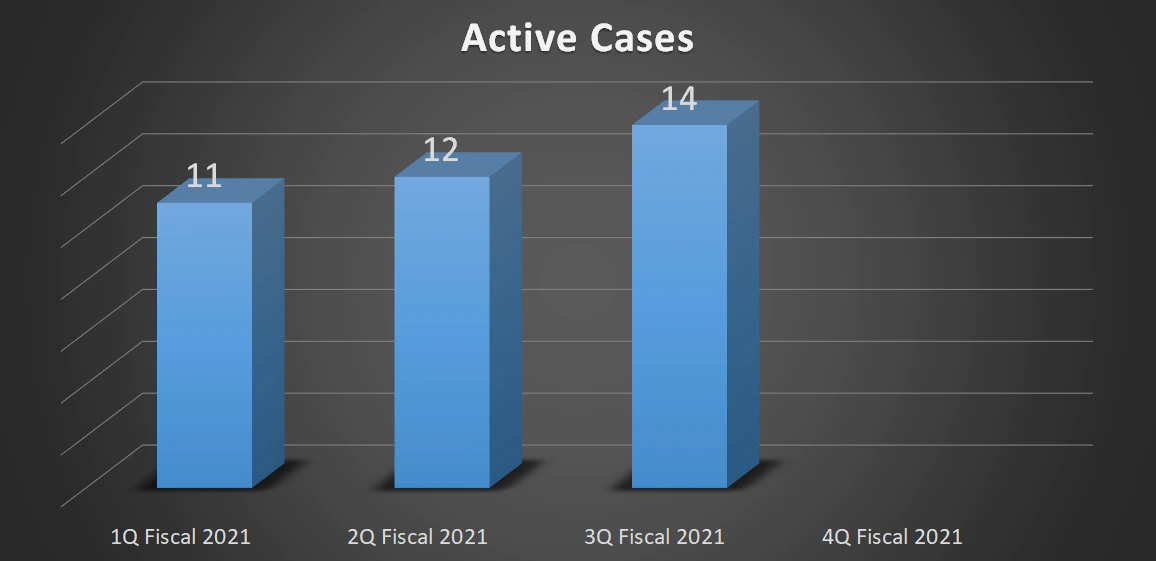
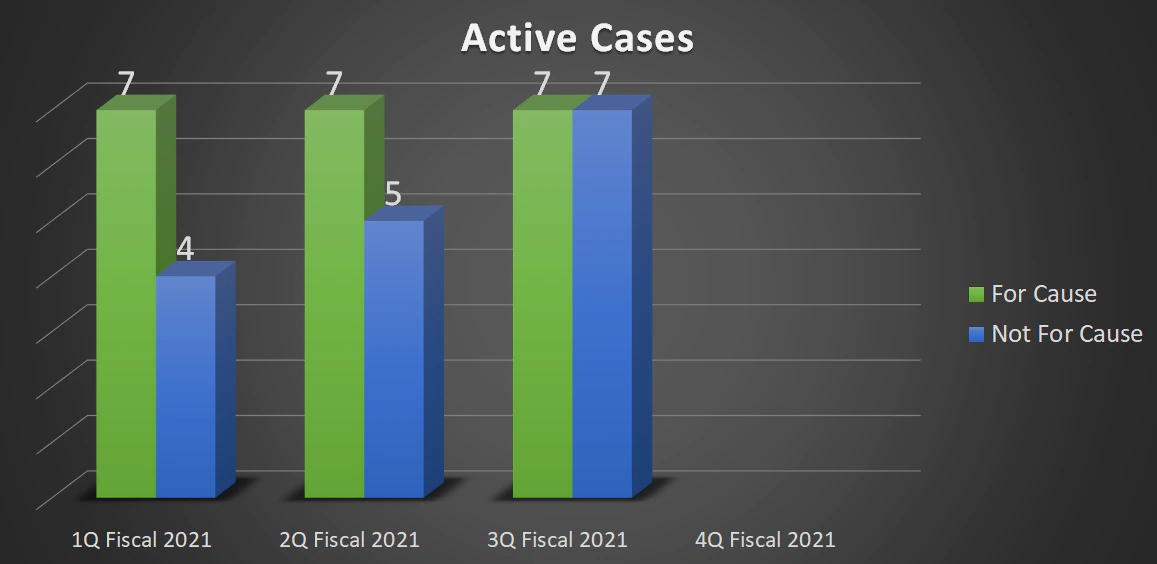
| Total Active Cases | 11 | 12 | 14 | - |
| For Cause | 7 | 7 | 7 | - |
| Not For Cause | 4 | 5 | 7 | - |
Data for the current year up to now:
| 2020 | 1Q Fiscal 2020 | 2Q Fiscal 2020 | 3Q Fiscal 2020 | 4Q Fiscal 2020 |
|---|---|---|---|---|
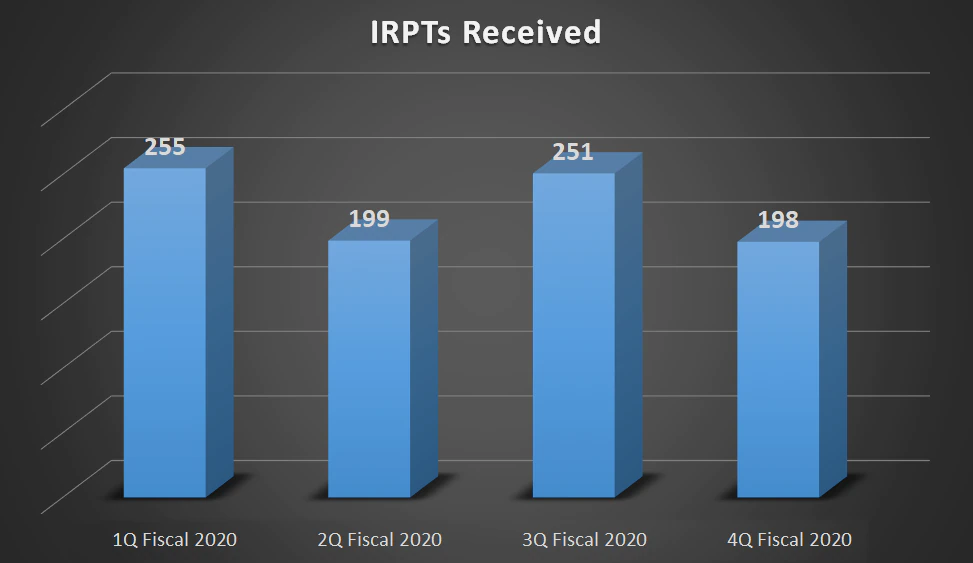
| IRPTs Received | 255 | 199 | 251 | 198 |
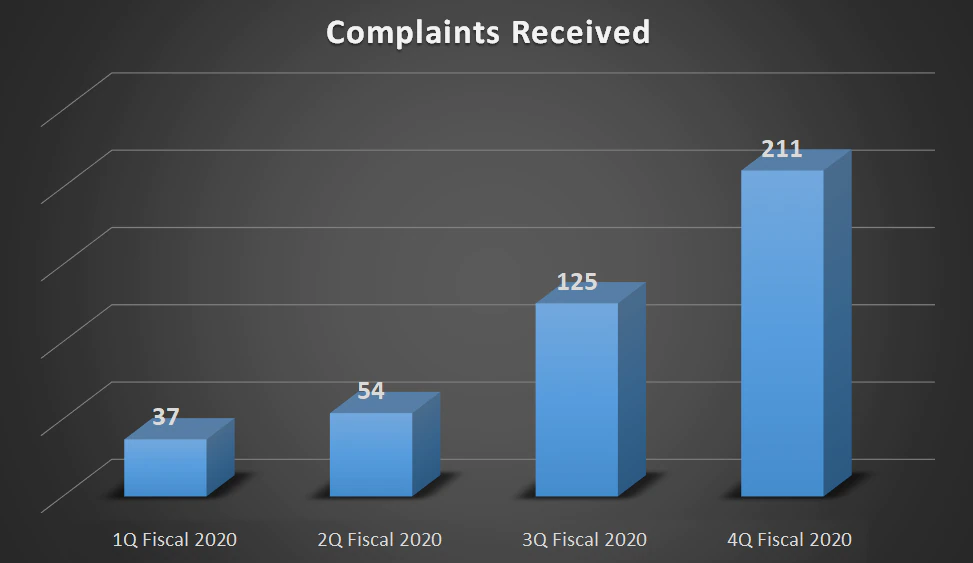
| Complaints Received | 37 | 54 | 125 | 211 |
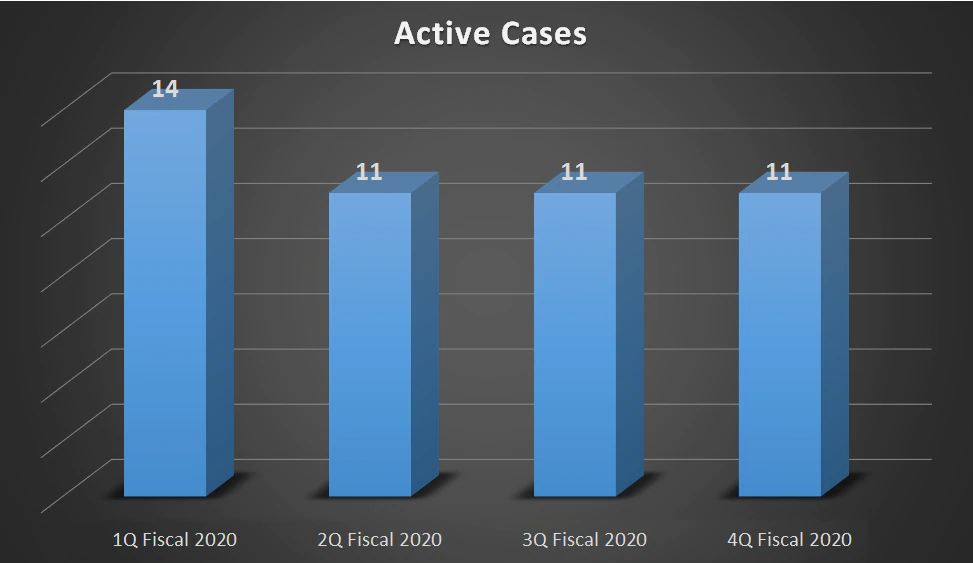
| Active Cases | 14 | 11 | 11 | 11 |
Data for 2020:
| 2019 | 1Q Fiscal 2019 | 2Q Fiscal 2019 | 3Q Fiscal 2019 | 4Q Fiscal 2019 |
|---|---|---|---|---|
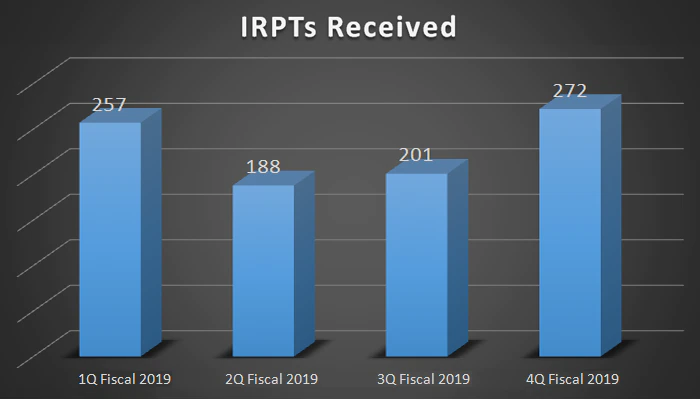
| IRPTs Received | 257 | 188 | 201 | 272 |
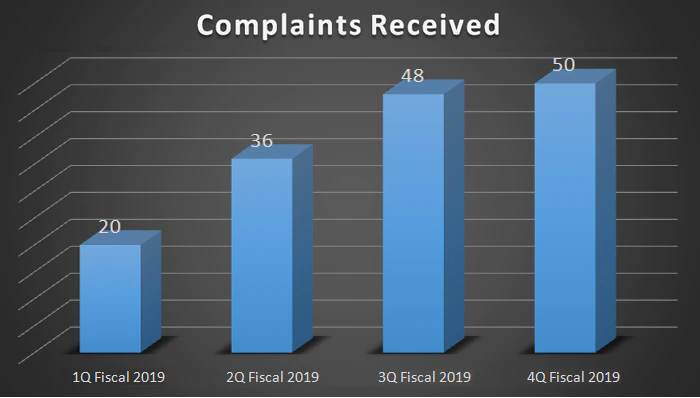
| Complaints Received | 20 | 36 | 48 | 50 |
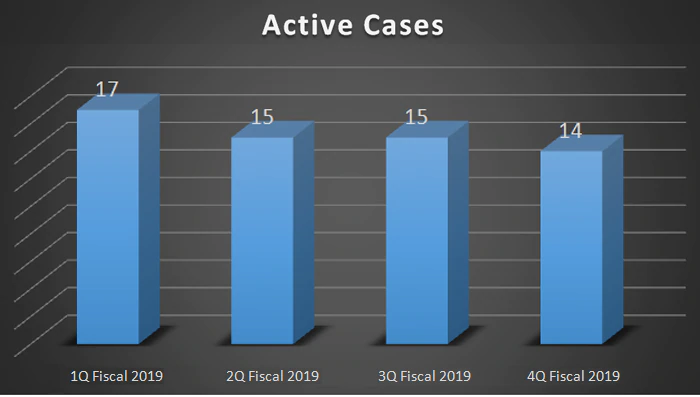
| Active Cases | 17 | 15 | 15 | 14 |
Data for 2019:
Definitions:
Incident Reports ("IRPTs") – Incident reports are notifications that OHRP receives from institutions to meet their regulatory obligation to report incidents of unanticipated problems involving risks to subjects or others; serious or continuing noncompliance with these regulations the requirements or determinations of the IRB; and suspensions or terminations of IRB approval. All incident reports are reviewed by DCO within 2 business days to identify incidents that may place research subjects at an increased risk and require immediate action from OHRP.
Full reports are complete initial reports that will not require a follow-up report.
Initial reports will require a follow-up report to be completed. This may be required for instances where the institution has not implemented a corrective action plan or has not completed gathering all information but is required to report incidents as required by the pre-2018 Requirements at 45 CFR 46.103(b)(5), and the 2018 Requirements at 45 CFR 46.108(a)(4).
Follow-up reports provide additional information to a previously submitted initial report.
Complaints – This data represents the total number of complaints OHRP receives. The majority of these complaints concern what complainants believe to be issues of non-compliance in human subjects research (HSR). However, for various reasons, once the terms of our regulations are applied, the object of the complaint does not constitute non-compliance, and OHRP acknowledges receipt of the complaint and takes no action. For example, some complaints are about research that is not covered by the regulations enforced by OHRP, and other complaints are from individuals for issues that do not pertain to the requirements of our regulations. Generally, the source of complaints sent to OHRP include, but are not limited to, research subjects and their family members, individuals involved in the conduct of research such as investigators and study coordinators, institutional officials, journalists, or media. OHRP takes all complaints seriously.
Active Cases – This data represents the number of OHRP cases that are ongoing and includes both for-cause and not-for-cause cases.
For-cause evaluations occur at OHRP's discretion, in response to OHRP's receipt of substantive written allegations or indications of noncompliance with the HHS regulations. Sources of such allegations or indications of noncompliance include, but are not limited to, research subjects and their family members, individuals involved in the conduct of research such as investigators and study coordinators, institutional officials, and research publications.
For-cause cases are those that are generally initiated as a result of allegations of non-compliance. Sources of such allegations or indications of noncompliance include, but are not limited to, research subjects and their family members, individuals involved in the conduct of research such as investigators and study coordinators, institutional officials, and research publications. OHRP may choose to use other mechanisms to address allegations or indications of noncompliance rather than conducting a for-cause evaluation. In general, information about these compliance activities are not provided to the public. For further details see OHRP’s guidance, "Compliance Oversight Procedures for Evaluating Institutions".
Not-for-cause compliance oversight evaluations are conducted in the absence of substantive allegations or indications of noncompliance. Institutions are selected for not-for-cause evaluation based on a range of considerations, including: (a) the volume of HHS-conducted or -supported research in which they are engaged; (b) whether they have a history of a relatively low level of reporting to OHRP under the requirements of HHS regulations at 45 CFR 46.103(b)(5); (c) the need to evaluate implementation of corrective actions following a previous for-cause compliance oversight evaluation; (d) geographic location; (e) status of accreditation by professionally recognized human subject protection program accreditation groups; and (f) status of recent human subject protection evaluations or audits by other regulatory agencies (such as the Food and Drug Administration).
For more information about compliances cases and how they are conducted, please visit OHRP’s website at: https://www.hhs.gov/ohrp/compliance-and-reporting/evaluating-institutions/index.html




