Overview
Please note: This lesson will take approximately 1 hour and 35 minutes to complete. Use the next and previous buttons to advance through the course. You will be able to print a completion certificate for your records at the end of this training. OHRP does not collect information about who accesses it.
Do not refresh your browser. Refreshing your browser will restart the lesson.
Purpose of this Lesson
This lesson will explain how the Common Rule regulations define “research” and “human subjects” and explain what it means to be exempt from the regulations. This lesson focuses on the Revised Common Rule (or 2018 Requirements) that became effective in 2018.
Lesson Overview
This lesson contains four parts:
- Part 1: Background of Human Subjects Research
- Part 2: Is the Activity Research?
- Part 3: Does the Research Involve Human Subjects?
- Part 4: Is the Human Subjects Research Exempt?
You will answer quiz questions throughout each part to test your knowledge. A correct response is required to advance in the lesson.
Learning Objectives
After completing this lesson, you will be able to:
- Identify if a certain activity meets the regulatory definition of research.
- Identify if research involves human subjects based on the regulatory definition.
- Determine whether a particular project is non-exempt human subjects research under the Common Rule.
Part 1: Background of Human Subjects Research
Go to Section: Introduction > The Concept of Non-exempt Human Subjects Research > Identifying Non-Exempt Human Subjects Research > Quiz Questions
Introduction
The Common Rule applies to human subjects research that is supported or conducted by a Common Rule agency. For research supported or conducted by the Department of Health and Human Services (HHS), the Office for Human Research Protections (OHRP) is the office with the authority to enforce the regulations. Many research institutions choose to apply the Common Rule to all of their human subjects research regardless of funding source.
This module focuses on the Revised Common Rule that became effective in 2018.
The Concept of Non-exempt Human Subjects Research

Even when funded by a Common Rule agency, not all research involving humans is required to follow the Common Rule. The Rule only applies to activities that qualify as human subjects research under the regulation and that do not qualify for an exemption. This is commonly referred to as non-exempt human subjects research.
Note that, in addition to the Common Rule (subpart A), non-exempt human subjects research funded by HHS must also comply with subparts B, C, & D of the regulations at 45 CFR 46. These subparts provide additional protections for certain special populations involved in research.
This module explains how the regulations define research and human subjects and explains what it means to be exempt from the regulations. Understanding these concepts is important to knowing when the regulations apply and when they do not.
Identifying Non-Exempt Human Subjects Research

To figure out whether a particular activity is non-exempt human subjects research under the Common Rule, ask the following three questions, in this order:
- Is the activity research according to the regulations?
- Does the research involve human subjects based on the definition in the regulations?
- Is the human subjects research exempt?
The determination of whether a research study is non-exempt human subjects research is usually made by an institution’s Human Research Protection Program (HRPP) or IRB office. In addition to applying the Common Rule’s basic protections for human subjects in research, the HRPP or IRB office also may ensure that the activity aligns with institutional policies, ethical guidelines, and other regulations and policies that might be relevant.
Question 1
Question 2
Question 3
Part 2: Is the Activity Research?
Go to Section: Defining Research > Categories of Activities Deemed Not to Be Research > Quiz Question > Determining When the Common Rule Requirements Apply
Defining Research
Let’s start with the first question: Is the activity research according to the regulations?
Not all work that we would colloquially call ‘research’ is considered to be research under the Common Rule. The Common Rule defines research as:
“a systematic investigation, including research development, testing, and evaluation,
designed to develop or contribute to generalizable knowledge.”
To decide if a certain activity meets the regulatory definition of research, consider:
- Whether the activity involves a systematic investigation.
- A systematic investigation generally refers to a methodical approach to the activity.
- It would likely involve a hypothesis, research question, and a plan to systematically collect and analyze data.
- A systematic investigation generally refers to a methodical approach to the activity.
- Whether the activity is designed to develop or contribute to generalizable knowledge.
- The systematic investigation adds information and contributes to generalizable knowledge in the field.
- Whether or how an investigator shares results with the scientific community is not the deciding factor for whether the activity was designed to develop or contribute to generalizable knowledge.
- For example, lots of information is published that comes from activities that do not meet the Common Rule’s definition of research. And sometimes results from research that meets the Common Rule definition never get published.
Categories of Activities Deemed Not to Be Research
The revised Common Rule also lists four specific types of activities that are deemed not to be research:
- Scholarly and journalistic activities that focus on information specifically about certain individuals.
- Certain public health surveillance activities.
- Certain activities solely for criminal justice or criminal investigative purposes.
- Certain operational activities in support of national security missions.
You can watch the video, “When Does the Common Rule Apply? Review of the Basics Under the Revised Rule ![]() ,” to review the revised Common Rule and how to determine when a research study is considered non-exempt human subjects research under the revised Rule. The presentation also includes a brief introduction of the exemptions.
,” to review the revised Common Rule and how to determine when a research study is considered non-exempt human subjects research under the revised Rule. The presentation also includes a brief introduction of the exemptions.
Question 1
![]()
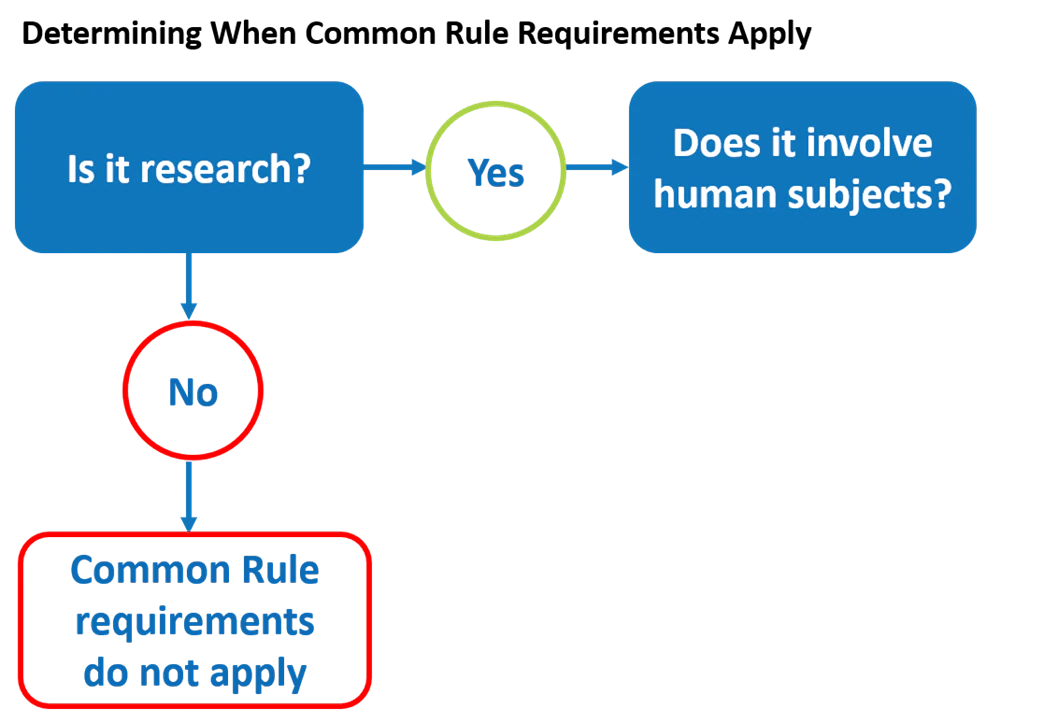
Determining When the Common Rule Requirements Apply
So, when deciding if a specific activity comes under the Common Rule,
First, ask whether it meets the regulatory definition for research—and remember to consider the four categories of activities deemed not to be research.
If the answer is “No,” then the Common Rule does not apply and, as a result, the activity does not have to be reviewed and approved by an IRB before starting. However, investigators should always check with their institution’s HRPP or IRB office to see whether there are institutional policies to follow even if the regulations don’t apply.
If, however, the answer to the first question is “Yes” – the activity does meet the regulatory definition of research, THEN ask the second question: Does the research involve human subjects?
Part 3: Does the Research Involve Human Subjects?
Go to Section: Defining Human Subject > Living Individuals > Identifying the Subject > Interaction and Intervention > Identifiable Private Information > Quiz Questions > Determining When the Common Rule Requirements Apply
Defining Human Subject
The revised Common Rule defines human subject as:
“a living individual about whom an investigator (whether professional or student) conducting research: (i) Obtains information or biospecimens through intervention or interaction with the individual, and, uses studies, or analyzes the information or biospecimens; or (ii) Obtains, uses, studies, analyzes, or generates identifiable private information or identifiable biospecimens.”
While there is a lot of detail in the definition of human subject, it generally boils down to this:
| If for the purpose of a research study… | Then… |
|---|---|
| An investigator:
|
The research likely involves human subjects. |
It is important to understand the key terms in this definition to determine when a research study involves human subjects according to the regulations.
Living Individuals
Human Subject: “a living individual about whom an investigator (whether professional or student) conducting research: (i) Obtains information or biospecimens through intervention or interaction with the individual, and, uses studies, or analyzes the information or biospecimens; or (ii) Obtains, uses, studies, analyzes, or generates identifiable private information or identifiable biospecimens.”

First of all, notice that it specifies living individuals. Therefore, for the purpose of the Common Rule, research that only uses materials from deceased persons would not be considered human subjects research.
Identifying the Subject
Human Subject: “a living individual about whom an investigator (whether professional or student) conducting research: (i) Obtains information or biospecimens through intervention or interaction with the individual, and, uses studies, or analyzes the information or biospecimens; or (ii) Obtains, uses, studies, analyzes, or generates identifiable private information or identifiable biospecimens.”

The phrase ‘about whom’ is important. A human subject is the person that the information is about, not necessarily the person providing the information. In the case of biospecimens, the human subject is the person from whom the specimen was taken.
For example:
| If… | Then… |
|---|---|
| An investigator gathers information about newborns by asking mothers questions only about the babies | Only the babies are the human subjects |
| The investigator asks for information only about the mothers | Only the mothers are human subjects |
| The investigator asks the mothers what they think about their babies’ behavior | Only the mothers are human subjects |
| The investigator asks the mothers how the babies behave and what the mothers think about their behavior | Both are human subjects |
Interaction and Intervention
Human Subject: “a living individual about whom an investigator (whether professional or student) conducting research: (i) Obtains information or biospecimens through intervention or interaction with the individual, and, uses studies, or analyzes the information or biospecimens; or (ii) Obtains, uses, studies, analyzes, or generates identifiable private information or identifiable biospecimens.”
 The terms ‘interaction’ and ‘intervention’ are central to the first part of this definition. Research involves human subjects when investigators interact or intervene with living individuals for the purpose of the research.
The terms ‘interaction’ and ‘intervention’ are central to the first part of this definition. Research involves human subjects when investigators interact or intervene with living individuals for the purpose of the research.
- Interactions occur when investigators communicate or have interpersonal contact with research participants, for example verbally, in writing, or electronically, to obtain information about them for the research.
- Interventions, on the other hand, include both physical procedures by which investigators collect information or biospecimens and manipulations of the subjects or the subjects’ environment for the purpose of the research.
- Examples of interventions include assigning subjects to take a particular drug in a clinical trial, asking subjects to complete a certain task for research purposes, and changing the background noise level to study how subjects’ stress levels vary.
Identifiable Private Information
Human Subject: “a living individual about whom an investigator (whether professional or student) conducting research: (i) Obtains information or biospecimens through intervention or interaction with the individual, and, uses studies, or analyzes the information or biospecimens; or (ii) Obtains, uses, studies, analyzes, or generates identifiable private information or identifiable biospecimens.”
 According to the second part of the definition, an activity can also be human subjects research if investigators have identifiable private information about research subjects or their identifiable biospecimens for the purpose of the research. The determining factor here is whether the information or biospecimens are identifiable—that is, the identity of the person is either known or can be readily ascertained by the investigator or the research team. Deciding whether information or biospecimens are identifiable is contextual and does not rely on a list of identifiers, like the list found in the HIPAA regulations.
According to the second part of the definition, an activity can also be human subjects research if investigators have identifiable private information about research subjects or their identifiable biospecimens for the purpose of the research. The determining factor here is whether the information or biospecimens are identifiable—that is, the identity of the person is either known or can be readily ascertained by the investigator or the research team. Deciding whether information or biospecimens are identifiable is contextual and does not rely on a list of identifiers, like the list found in the HIPAA regulations.
Note, also, that the researchers may or may not have interacted or intervened with the subject at all – for example, they might use leftover blood samples from clinical tests; but if the blood sample is identifiable, then the person is considered to be a human subject.
Question 1
Question 2
Question 3
![]()
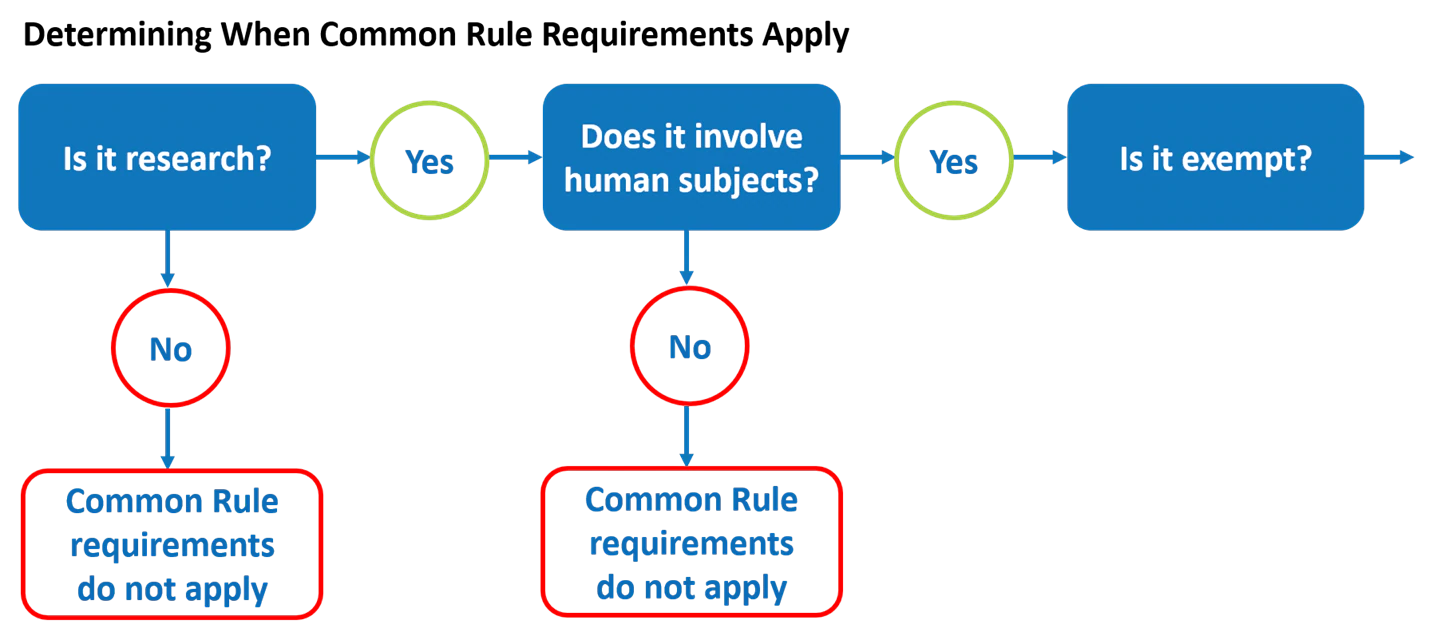
Determining When the Common Rule Requirements Apply
Apply this definition of “human subjects” to your research to determine whether your research study constitutes human subjects research under the Common Rule. If the answer is “no,” then the Common Rule does not apply.
If, on the other hand, the answer to this second question is “yes,” and it is human subjects research, then you go on to the third question: Is it exempt?
Part 4: Is the Human Subjects Research Exempt?
Go to Section: Could the Human Subjects Research Be Exempt? > Exempt Human Subjects Research > Common Rule Exemptions Videos > Quiz Question > Determining When the Common Rule Requirements Apply
Could the Human Subjects Research Be Exempt?
There are eight exemption categories listed in the revised Common Rule. If all of the activities in a human subjects research study meet the criteria for one or more of these exemption categories, the study is exempt from the Common Rule requirements for oversight. This means, for example, that the research does not need to undergo initial or continuing IRB review for approval as required by the regulations.

The Common Rule does not specify who can make determinations about exemptions. Most institutions require that investigators submit proposed research to the institution’s HRPP or IRB office for the determination about whether it meets the criteria for an exemption. Additionally, certain exemptions require a “limited IRB review” to determine that specific conditions are met for the exemption to apply.
Exempt Human Subjects Research
Human subjects research projects that have been determined to be exempt from the regulations can generally proceed without having to comply with the regulatory requirements.

One thing to remember, however, is that if investigators make changes to the research at a later time, they should check with their institution’s HRPP or IRB office to make sure that the research still meets the exemption criteria. If the changes cause the research study to no longer meet the criteria for exemption, then the research is no longer exempt and must comply with the regulatory requirements and undergo IRB review. Investigators should work closely with their HRPP or IRB office to avoid surprises like this that could affect the progress of their research.
Common Rule Exemptions Videos
Video 1 – Overview of Changes to Exemptions in the Revised Common Rule (Focusing on Exemptions 1, 2, 3, and 5) (26:06)
Watch this video to learn about the exemptions 1, 2, 3, and 5 in the revised Common Rule.
Video 2 – Regulatory Options for Secondary Research with Private Information and Biospecimens Part 1 (Including Discussion of Exemptions 4, 7, and 8) (24:59)
Watch this video to learn about exemptions 4, 7, and 8, the concept of secondary research and how the exemptions provide flexibility for conducting secondary research under the revised Common Rule.
Question 1
![]()
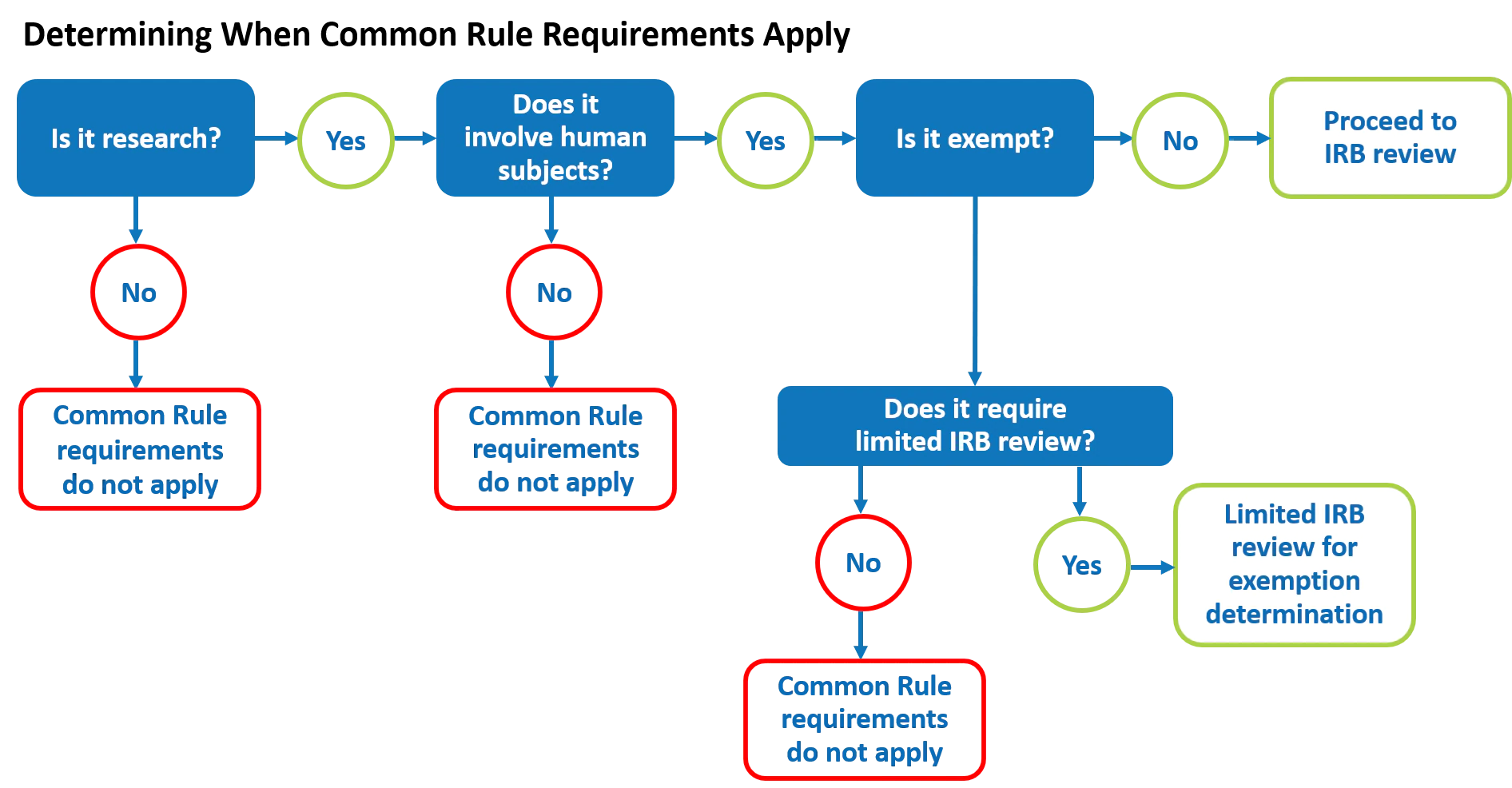
Determining When the Common Rule Requirements Apply
Human subjects research studies that do not qualify for an exemption are referred to as non-exempt human subjects research. Unless there is a Secretarial waiver, they must comply with the Common Rule requirements, including IRB review and approval, before the research can begin. For non-exempt cooperative research studies involving multiple institutions, the review would generally be done by a single IRB.
Conclusion
Go to Section: Wrap Up > Completion Certificate
Wrap Up
This module explained the process of determining whether a research project meets the criteria for being non-exempt human subjects research under the Common Rule. Remember that if it doesn’t satisfy the regulatory definition of either research or human subject, or if all of the activities in the human subjects research meet the criteria for one or more of the exemptions, then the Common Rule requirements do not apply to the project, but investigators may still be subject to any institutional policies that are in place. Investigators should work with their institution’s Human Research Protection Program (HRPP) or IRB office to find answers and determine how to proceed.

Congratulations!
You have completed OHRP’s learning module:
Lesson 2: What is Human Subjects Research?
OHRP does not collect information about who completes this training. Please fill out the information below and print this page for your records.
Name:
Date:





