Date: May 26, 2005
Scope:
This document provides guidance on the HHS 45 CFR 46.407 review process ("407 review process") as required under subpart D of HHS Protection of Human Subjects Regulations at 45 CFR part 46. In particular, the Office for Human Research Protections (OHRP) offers guidance on the following topics:
(1) IRB findings necessary to submit a protocol to OHRP for 407 consideration and/or review;
(2) steps in the submission process;
(3) OHRP's response to submissions;
(4) the schedule and details for 407 panel review; and
(5) potential outcomes of the 407 review process.
This guidance applies to HHS- conducted or -supported research.
NOTE: Protocols meeting the conditions of 45 CFR 46.407 also may be subject to Food and Drug Administration (FDA) regulations under 21 CFR 50.54 if the protocols involve a clinical investigation of an FDA-regulated product. Other protocols may be subject to FDA regulation at 21 CFR 50.54 but not subject to HHS regulations at 45 CFR 46.407. Although this guidance document briefly addresses steps that would be taken when FDA regulations apply, it is focused on the 407 review process for research solely within OHRP's purview. The reader is advised to consult with FDA in the event that the review process falls within FDA's regulatory purview. [FDA Contact Information: Office of Pediatric Therapeutics, Office of the Commissioner, Food and Drug Administration, 5600 Fishers Lane, Room 48-44, HFG-2, Rockville, MD 20857, (301) 827-1996.]
Target Audience: Institutions, Institutional Review Boards (IRBs), investigators, and HHS funding agencies.
General Regulatory Background:
HHS regulations at 45 CFR part 46 include subpart D, Additional Protections for Children Involved as Subjects in Research. All studies involving children, conducted or supported by HHS, which are not otherwise exempt, require IRB review in accordance with the provisions of subpart D. If an institution's IRB does not believe the proposed research meets the requirements of 45 CFR 46.404, 46.405, or 46.406 of subpart D (described below), but finds and documents that the research presents a reasonable opportunity to further the understanding, prevention, or alleviation of a serious problem affecting the health or welfare of children (in accordance with HHS regulations at 45 CFR 46.407(a)), the IRB or other appropriate institutional official may submit the protocol and supporting materials to OHRP for HHS consideration under the provisions of 45 CFR 46.407(b). Before submitting a protocol to OHRP, the IRB must determine that, in addition to meeting the requirements of 45 CFR 46.407(a) and other applicable sections of subpart D, the proposed research also meets all of the requirements of 45 CFR part 46, subpart A.
HHS will consult with a panel of experts under 46.407 only when the proposed research is conducted or supported by HHS. Note that if an institution has elected in its assurance to apply all of the subparts of 45 CFR part 46 to all of its human subjects research regardless of the source of support, and the IRB finds that the proposed research meets the conditions for review under 46.407, the IRB is not required to submit the protocol to OHRP for review if the research under consideration is not supported by HHS. In such cases, OHRP recommends that the institution consult with appropriate officials at the relevant federal agency or department supporting the research. When such research is supported by a non-federal sponsor, OHRP recommends that the institution consider convening an independent panel of experts in pertinent disciplines (e.g., science, medicine, education, ethics, law) and provide an opportunity for review and comment by the local community where the research is to be conducted before deciding whether to proceed with the research.
Subpart D permits IRBs to approve three categories of research involving children as research subjects:
45 CFR 46.404 - Research not involving greater than minimal risk to the children. To approve this category of research, the IRB must make the following determinations:
- the research presents no greater than minimal risk to the children; and
-
adequate provisions are made for soliciting the assent of the children and the permission of their parents or guardians, as set forth in HHS regulations at 45 CFR 46.408.
45 CFR 46.405 - Research involving greater than minimal risk but presenting the prospect of direct benefit to the individual child subjects involved in the research. To approve research in this category, the IRB must make the following determinations:
- the risk is justified by the anticipated benefits to the subjects;
- the relation of the anticipated benefit to the risk presented by the study is at least as favorable to the subjects as that provided by available alternative approaches; and
-
adequate provisions are made for soliciting the assent of the children and the permission of their parents or guardians, as set forth in HHS regulations at 45 CFR 46.408.
45 CFR 46.406 - Research involving greater than minimal risk and no prospect of direct benefit to the individual child subjects involved in the research, but likely to yield generalizable knowledge about the subject's disorder or condition. In order to approve research in this category, the IRB must make the following determinations:
- the risk of the research represents a minor increase over minimal risk;
- the intervention or procedure presents experiences to the child subjects that are reasonably commensurate with those inherent in their actual, or expected medical, dental, psychological, social, or educational situations;
- the intervention or procedure is likely to yield generalizable knowledge about the subject's disorder or condition which is of vital importance for the understanding or amelioration of the disorder or condition; and
-
adequate provisions are made for soliciting the assent of the children and the permission of their parents or guardians, as set forth in HHS regulations at 45 CFR 46.408.
A fourth category of research requires a special level of HHS review beyond that provided by the IRB.
If the IRB believes that the research does not meet the requirements of 45 CFR 46.404, 46.405, or 46.406, but finds that it presents a reasonable opportunity to further the understanding, prevention, or alleviation of a serious problem affecting the health or welfare of children, it may refer the protocol to HHS for review. The research may proceed only if the Secretary, HHS, or his or her designee, after consulting with a panel of experts in pertinent disciplines (e.g., science, medicine, education, ethics, law) and following an opportunity for public review and comment, determines either: (1) that the research in fact satisfies the conditions of 45 CFR 46.404, 46.405, or 46.406, or (2) the following:
- the research presents a reasonable opportunity to further the understanding, prevention, or alleviation of a serious problem affecting the health or welfare of children;
- the research will be conducted in accordance with sound ethical principles; and
- adequate provisions are made for soliciting the assent of children and the permission of their parents or guardians, as set forth in HHS regulations at 45 CFR 46.408.
If the research involves a product that is FDA-regulated, FDA's regulatory requirements at 21 CFR 50.54 must also be met.
Guidance:
What Findings Should IRBs Make before Submitting a Protocol to HHS for 407 Consideration and/or Review?
The IRB must determine that the protocol does not meet the conditions for approval of research under HHS regulations at 45 CFR 46.404, 46.405, or 46.406 as described above.
Before submitting a request to OHRP for a review under the 407 process, the IRB also must determine that the proposed research and the parental permission/assent forms comply with all regulatory requirements and are otherwise approvable (i.e., meet the regulatory requirements under 45 CFR 46.111, 46.408, and 46.409), with the exception of the need for review under the 407 process. Any modifications to the protocol or parental permission/assent forms required by the IRB to comply with 45 CFR 46.111, 46.408, or 46.409 must be made by the principal investigator before materials are submitted to OHRP for consideration.
What are the Steps in the Referral Process?
1. IRB Request for 407 Review
Once an IRB determines that a protocol does not meet the requirements of 46.404, 46.405, or 46.406 for approval of research, but does meet the requirements for review under 45 CFR 46.407(a), the institution or the IRB may request that OHRP, on behalf of the Secretary, HHS, conduct a 46.407 review. In order for OHRP to determine whether a 407 review should proceed, the institution must submit the following documents/information to OHRP in both written and electronic (if available) forms:
- IRB documentation of required findings under 45 CFR 46.407Cthat the proposed research does not meet the requirements of 46.404, 46.405, or 46.406 but presents a reasonable opportunity to understand, prevent, or alleviate a serious problem affecting the health or welfare of children.
- Institution name, institution assurance number, and IRB name.
- Institutional contact's name, title, phone number, fax number, mailing address, and email address.
- Title of protocol, and name of principal investigator(s).
- HHS application number and name of funding agency.
- Relevant HHS grant application or proposal.
- Most current version of protocol and grant application submitted to and reviewed by the IRB and modified by the principal investigator if required by the IRB.
- Most current version of parental permission/assent documents submitted to and reviewed by the IRB and modified by the principal investigator if required by the IRB.
- Relevant IRB minutes and correspondence.
Hard copy versions of the materials should be sent to:
Office for Human Research Protections
Department of Health and Human Services
1101 Wootton Parkway, Suite 200
Rockville, MD 20852
OHRP will provide the submitting institution with instructions for transmitting documents electronically.
OHRP will notify the relevant HHS funding agency that it has received a request for a 407 review of a protocol. OHRP will request information from the agency about the outcome of the merit review of the research (i.e., the summary statement of the relevant Scientific Review Group or the evaluation of the proposal).
2. OHRP's Initial Evaluation of the Request for 407 Review
After receiving the materials listed above, OHRP will review the protocol and supporting information to evaluate whether the conditions for review under 46.407 by an expert panel have been met. In the process of this review OHRP may request additional information or clarification from the IRB. In all cases, OHRP will confer with FDA to determine if FDA regulations under 21 CFR 50.54 also apply. In cases where FDA regulations do apply, OHRP has delegated its authority to FDA to convene a panel of experts to both review the research and to advise the Secretary (see discussion at the end of this guidance). In all cases, OHRP will notify the IRB, the investigator, and the funding agency in writing of its evaluation, which generally would include one of the following outcomes:
- the information submitted to OHRP is insufficient to enable OHRP to evaluate whether a review of the proposed research under HHS regulations under 45 CFR 46.407 is appropriate; or
- the research might be approvable under 46.404, 46.405, or 46.406, and the IRB should reconsider its evaluation of the protocol; or
- the research fulfills the criteria for consideration by HHS under the provisions of 46.407 and OHRP will initiate the review process; or
- the research fulfills the criteria for consideration by HHS under the provisions of 46.407, and because FDA regulations also apply, FDA will convene a panel of experts in coordination with OHRP to review the protocol.
When OHRP has sole responsibility for the 407 review process (i.e., FDA regulations do not apply), the schedule described below is initiated.
3. OHRP Schedule for 407 Review Process When FDA Regulations Do Not Apply
If OHRP has determined that the conditions for 407 review have been met and that FDA regulations do not apply, OHRP will identify a panel of experts in pertinent disciplines (e.g., science, medicine, education, ethics, law) and relevant child advocates to review the protocol. Potential experts will undergo an initial screening for conflicts of interest. Potential experts will be informed that they will be asked to provide individual written recommendations and that their reports, as well as their identities, will be publicly available during the public review and comment period.
As noted above, OHRP will notify the funding agency that a referral for a 407 review has been received and accepted. If the research is a multi-site protocol, the funding agency may consider the implications of the 407 review process on the conduct of the research at the other sites and would have the option of delaying or suspending subject enrollment at these other sites pending the outcome of the 407 review. If necessary and appropriate, OHRP may also invoke its regulatory authority to delay or suspend such research at other sites pending the outcome of the 407 review process.
If the relevant grant application or proposal contains multiple protocols and the IRB or institution refers one protocol to HHS for 407 review, then the awardee institution cannot certify that the entire grant application or proposal has received IRB approval. In such situations, the funding agency could issue a restricted award so that the IRB-approved protocols within the grant can go forward.
OHRP will request written permission from the institution, the IRB, and the principal investigator to allow public review of the grant application, protocol application, parental permission/assent documents, and relevant IRB records. If permission is granted to post the relevant information, OHRP will solicit public review and comment on the proposed research in accordance with the requirements of HHS regulations at 45 CFR 46.407(b). Absent permission to release this information, OHRP may determine that there is not enough information available to the public to allow meaningful comment on the proposed research. Because of OHRP's obligation under the regulations to request public comments before HHS support for the research can be approved, this would effectively halt the 407 review process and the research could not proceed.
Notice of Meeting and Request for Public Comments
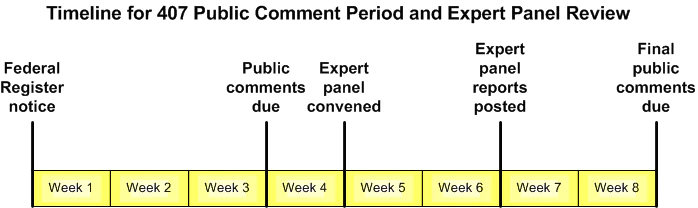
A request for written comments will be published in the Federal Register, including an Internet link to the protocol, relevant sections of grant applications, parental permission and assent documents, and relevant excerpts from the IRB minutes and correspondence. In most cases, the total public comment period will last for 60 days after the Federal Register notice has been published. [See timeline for public comment period.]
In addition to requesting public comments on the posted materials, the Federal Register notice will also include the following:
- the date and location of the expert panel meeting (to be held a minimum of four weeks after the notice is posted);
- indication that the panel meeting will be open to the public and that the public will be given an opportunity to comment at the panel meeting;
- a note that written comments on posted materials must be submitted at least one week before the day of the panel meeting to be considered by the panelists (which will allow the public three weeks to comment on posted materials);
- indication that the panelists' reports/recommendations (see below) will be posted two weeks after the panel meets; and
- an invitation to provide comments for up to 30 days after the meeting of the convened panel for review and consideration by OHRP, a summary of which will be transmitted to the Secretary, HHS.
4. Convened OHRP 407 Panel Meeting
All 407 panel meetings will be open to the public. After the convened panel discussion occurs and public comments are received, each panel member will write an independent recommendation as to whether the protocol should proceed, proceed with modifications, or not proceed.
Panel reports will be posted on the OHRP website for informational purposes. The public may continue to provide comments on the protocol and other posted materials for 30 days after the date of the convened panel meeting.
5. Conclusion of the OHRP Process
Based on panel deliberations, reports, public comments, and its own analysis, OHRP will develop a recommendation within 90 days of the convened panel meeting. Possible outcomes of the 407 review process include:
- HHS approves support of the research as submitted;
- HHS approves support of the research, but with required and/or recommended modifications; or
- HHS disapproves support of the research.
The Assistant Secretary for Health, on behalf of the Secretary, will make the final determination regarding whether the research may be supported based on all available material and OHRP's recommendation. Once this determination has been made, the IRB Chair, the investigator, the institution, and the funding agency will be immediately informed of the decision, which will be posted on OHRP's website. The posting will include OHRP's recommendation to the Assistant Secretary. If the research is a multi-site protocol, and a determination is made by HHS not to support the research, OHRP will notify the funding agency that the research described in the disapproved protocol must be terminated at all sites.
If the Secretary's determination is that the protocol should proceed after modifications, the investigator must modify the research proposal, parental permission/assent forms, and other documents as appropriate, and submit the revisions to the IRB for review and approval. The IRB or other appropriate institutional official must then submit the approved revised protocol to OHRP for final concurrence before the research can proceed. Once OHRP reviews the revisions, it will communicate the outcome of the process to the IRB, the principal investigator, the institution, and the funding agency.
407 Review Process When FDA Regulations Apply
In the event that both OHRP and FDA regulations apply, FDA will issue a notice in the Federal Register about its schedule for review and receipt of public comments. In cooperation with OHRP, FDA will convene the Pediatrics Ethics Subcommittee of its Pediatric Advisory Committee to review the protocol. The Pediatrics Ethics Subcommittee will transmit a consensus report to the FDA Pediatric Advisory Committee, which will then make its final recommendations to the FDA Commissioner. The Pediatric Advisory Committee recommendations will be transmitted to the FDA Commissioner through the Office of Pediatric Therapeutics, which will comment on the recommendations in an accompanying transmittal memorandum. The FDA Commissioner's decision as to whether the protocol may proceed will then be transmitted along with OHRP's recommendation to the Secretary, HHS, through the Assistant Secretary for Health. OHRP's recommendation to the Assistant Secretary for Health will be based on the findings of the Pediatrics Advisory Committee, the FDA Commissioner, public comments, and OHRP internal review.
If you have specific questions about how to apply this guidance, please contact OHRP by phone at (866) 447-4777 (toll-free within the U.S.), (301) 496-7005, or by e-mail at ohrp@hhs.gov.
Previous 407 reviews are available in the OHRP Archive.




