
OHRP’s guidance provides instructions on reporting incidents to OHRP, for HHS conducted or supported human subjects research. To report incidents to other Common Rule agencies, please contact the funding agency directly. A list of contacts for Common Rule agencies is provided below. OHRP does not submit incident reports to other Common Rule agencies.
- Common Rule Agency Contacts
- Print the 2011 Reporting Guidance
- Mini-Tutorial videos on Reporting to OHRP
Submission of the OHRP Incident Report Form is required for any incident report made to OHRP in accordance with 45 CFR part 46. The form and the associated instructions may be found at the links below:
- OHRP Incident Report Form - To avoid compatibility issues with different browsers, download the form prior to completion. Adobe is recommended.
- OHRP Incident Report Form Instructions
Guidance on Reporting Incidents to OHRP (2011)
NOTE: THIS GUIDANCE REPLACES OHRP'S MAY 27, 2005 GUIDANCE ENTITLED "GUIDANCE ON REPORTING INCIDENTS TO OHRP". This guidance has been updated to clarify what information regarding serious or continuing noncompliance by the institutional review board needs to be reported, to include an e-mail address to report incidents to OHRP, and to update OHRP's contact information.
This guidance represents OHRP's current thinking on this topic and should be viewed as recommendations unless specific regulatory requirements are cited. The use of the word must in OHRP guidance means that something is required under HHS regulations at 45 CFR part 46. The use of the word should in OHRP guidance means that something is recommended or suggested, but not required. An institution may use an alternative approach if the approach satisfies the requirements of the HHS regulations at 45 CFR part 46. OHRP is available to discuss alternative approaches at 240-453-6900 or 866-447-4777.
Date: June 20, 2011
Scope:
This document provides guidance about procedures institutions may use to file incident reports with OHRP. Incident reports include reports of unanticipated problems involving risks to subjects or others; serious or continuing noncompliance with Department of Health and Human Services (HHS) regulations at 45 CFR part 46 or the requirements or determinations of the institutional review board (IRB); and suspension or termination of IRB approval. In particular, OHRP offers guidance on the following topics:
- Applicability of incident reporting requirements;
- Information to be included in incident reports;
- Time frame for reporting incidents;
- OHRP focus on corrective actions when reviewing incident reports;
- OHRP's response to incident reports;
- Where to send incident reports; and
- Additional guidance.
Target Audience: IRBs, institutional officials and institutions that may be responsible for review, oversight, or conduct of human subjects research covered by an OHRP-approved assurance.
Regulatory Background:
HHS regulations at 45 CFR 46.103(a) and (b)(5) require that institutions have written procedures to ensure that the following incidents related to regulatory requirements pertaining to research conducted under an OHRP- approved assurance are promptly reported to OHRP:
- Any unanticipated problems involving risks to subjects or others;
- Any serious or continuing noncompliance with this policy or the requirements or determinations of the IRB; and
- Any suspension or termination of IRB approval.
Guidance:
In general, these reporting requirements apply to all nonexempt human subjects research that is:
- conducted or supported by HHS;
- conducted or supported by any non-HHS federal department or agency that has adopted the Common Rule and is covered by a Federalwide Assurance (FWA) determined to be appropriate for such research; or
- covered by an FWA, regardless of funding source.
Federal departments or agencies other than HHS that have adopted the Common Rule may determine that the FWA is not appropriate for certain research that they conduct or support. OHRP notes that these incident reporting requirements are not applicable to such research. In such cases, the institution should contact the non-HHS department or agency that supports the research about reporting requirements. See the decision chart below.
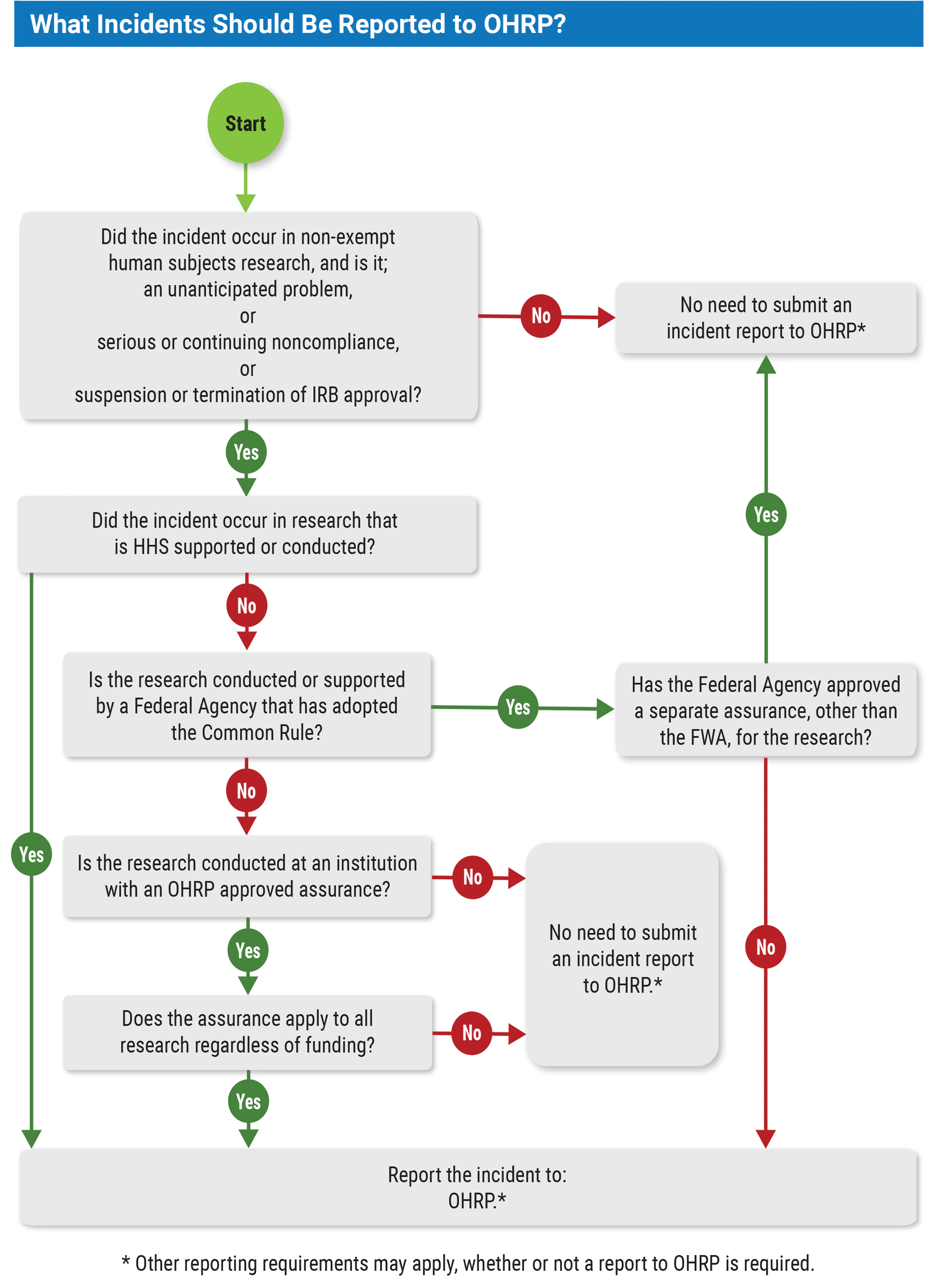
To fulfill the regulatory requirements for reporting incidents, OHRP would consider it acceptable for an institution to comply with written procedures specifying that the following information be included in an incident report submitted to OHRP:
A. For unanticipated problems involving risks to subjects or others:
- Name of the institution (e.g., university, hospital, foundation, school, etc) conducting the research;
- Title of the research project and/or grant proposal in which the problem occurred;
- Name of the principal investigator on the protocol;
- Number of the research project assigned by the IRB and the number of any applicable federal award(s) (grant, contract, or cooperative agreement);
- A detailed description of the problem; and
- Actions the institution is taking or plans to take to address the problem (e.g., revise the protocol, suspend subject enrollment, terminate the research, revise the informed consent document, inform enrolled subjects, increase monitoring of subjects, etc.).
B. For serious or continuing noncompliance:
- Name of the institution (e.g., university, hospital, foundation, school, etc) conducting the research;
- Title of the research project and/or grant proposal in which the noncompliance occurred, or, for IRB or institutional noncompliance, the IRB or institution involved;
- Name of the principal investigator on the protocol, if applicable;
- Number of the research project assigned by the IRB and the number of any applicable federal award(s) (grant, contract, or cooperative agreement);
- A detailed description of the noncompliance; and
- Actions the institution is taking or plans to take to address the noncompliance (e.g., educate the investigator, educate all research staff, educate the IRB or institutional official, develop or revise IRB written procedures, suspend the protocol, suspend the investigator, conduct random audits of the investigator or all investigators, etc.).
C. For suspension or termination:
- Name of the institution (e.g., university, hospital, foundation, school, etc.) conducting the research;
- Title of the research project and/or grant proposal that was suspended or terminated;
- Name of the principal investigator on the protocol;
- Number of the research project assigned by the IRB that was suspended or terminated and the number of any applicable federal award(s) (grant, contract, or cooperative agreement);
- A detailed description of the reason for the suspension or termination; and
- The actions the institution is taking or plans to take to address the suspension or termination (e.g., investigate alleged noncompliance, educate the investigator, educate all research staff, require monitoring of the investigator or the research project, etc.)
The regulations at 45 CFR 46.103(a) and (b)(5) do not specify a time frame for reporting, except to say this must be done "promptly." For a more serious incident, this may mean reporting to OHRP within days. For a less serious incident, a few weeks may be sufficient. It may be appropriate to send an initial report, and indicate that a follow-up or final report will follow by the earlier of:
- a specific date; or
- when an investigation has been completed or a corrective action plan has been implemented.
When reviewing a report of an unanticipated problem, OHRP assesses most closely the adequacy of the actions taken by the institution to address the problem. Likewise, when reviewing reports of non-compliance or suspension or termination of IRB approval, OHRP assesses most closely the adequacy of the corrective actions taken by the institution. In particular, OHRP assesses whether or not the corrective actions will help ensure that the incident will not happen again, with the investigator or protocol in question, with any other investigator or protocol, or with the IRB. Therefore, OHRP recommends that, when appropriate, corrective actions be applied institution-wide.
After receiving and evaluating an incident report from an institution, OHRP will respond in writing and will either state that the report was adequate or request additional information. For questions on reporting, please contact the Director of the Division of Compliance Oversight, (240) 453-6900 or (866) 447-4777.
Please send reports (PDF or Word documents preferred) to the following email address:
IRPT.OS@hhs.gov




