Building Proactivity into FDA Communication and Decision Making
Executive Summary
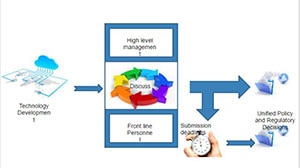 The Center for Devices and Radiological Health (CDRH) within the FDA regulates many high-tech, fast-moving medical device sectors. Medical products or high-tech trends in medical devices often come to the attention of CDRH once they are about to be released on the market. Communication of information across the organization then becomes critical, especially with short, regulatory timelines.
The Center for Devices and Radiological Health (CDRH) within the FDA regulates many high-tech, fast-moving medical device sectors. Medical products or high-tech trends in medical devices often come to the attention of CDRH once they are about to be released on the market. Communication of information across the organization then becomes critical, especially with short, regulatory timelines.
Emerging technology trends can, and indeed in the past have, disrupted existing regulatory paradigms before the FDA is prepared to respond. Recent examples of disruptive technologies are mobile medical apps, additive manufacturing (3d printing), and nanotechnology. These types of market disruptions are critically important and beneficial to the future of health and health care in this country and worldwide. However, from an organizational standpoint, this same disruptive nature makes it difficult for government, and the FDA in particular, to understand the potential risks involved.
Currently, there is no coordinated system to consolidate, process, and disseminate information on disruptive technologies that cut across product lines. Silos of expertise can lead to inconsistent decision-making and delayed policy decisions.
The Innovation: This team designed a web platform that would allow for FDA staff to create ad hoc interest groups. Others in the organization would then be able to search the database of created groups and commit a portion of their time to helping drive the conversation or achieve whatever goal the group sets out to accomplish.
The team built on a product called midas, an open source effort led by 18F but for which the State Department and HHS have been early contributors.
A project supported by the: HHS Ignite Accelerator
Team Members
James Coburn (Project Lead), FDA
Jessica Hernandez, FDA
Jennifer Kelly, FDA
David Hwang, FDA
Kathryn O’Callaghan, FDA
Milestones
May 2014: Project selected into the HHS Ignite Accelerator
June 2014: Time in the Accelerator began
September 2014: Time in the Accelerator ended
Project Sponsor
Victor Krauthamer, Division Director, Office of Science and Engineering Laboratories, Center for Devices and Radiological Health, Food and Drug Administration


