Fact Sheet: Explaining Operation Warp Speed
What's the goal?
Operation Warp Speed's goal is to produce and deliver 300 million doses of safe and effective vaccines with the initial doses available by January 2021, as part of a broader strategy to accelerate the development, manufacturing, and distribution of COVID-19 vaccines, therapeutics, and diagnostics (collectively known as countermeasures).
How will the goal be accomplished?
By investing in and coordinating countermeasure development, OWS will allow countermeasures such as a vaccine to be delivered to patients more rapidly while adhering to standards for safety and efficacy.
Who's working on Operation Warp Speed?
OWS is a partnership among components of the Department of Health and Human Services (HHS), including the Centers for Disease Control and Prevention (CDC), the National Institutes of Health (NIH), and the Biomedical Advanced Research and Development Authority (BARDA), and the Department of Defense (DoD). OWS engages with private firms and other federal agencies, including the Department of Veterans Affairs. It will coordinate existing HHS-wide efforts, including the NIH's Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) partnership, NIH's Rapid Acceleration of Diagnostics (RADx) initiative, and work by BARDA.
What's the plan and what's happened so far?
Development
To accelerate development while maintaining standards for safety and efficacy, OWS has been selecting the most promising countermeasure candidates and providing coordinated government support.
Protocols for the demonstration of safety and efficacy are being aligned, which will allow the trials to proceed more quickly, and the protocols for the trials will be overseen by the federal government, as opposed to traditional public-private partnerships, in which pharmaceutical companies decide on their own protocols. Rather than eliminating steps from traditional development timelines, steps will proceed simultaneously, such as starting manufacturing of the vaccine at industrial scale well before the demonstration of vaccine efficacy and safety as happens normally. This increases the financial risk, but not the product risk.
Select actions to support OWS vaccine and therapeutic development so far include:
March
March 30: HHS announced $456 million in funds for Johnson & Johnson's (Janssen) candidate vaccine. Phase 1 clinical trials began in Belgium on July 24th and in the U.S on July 27th. Janssen's large-scale Phase 3 clinical trial began on September 22, 2020, making them the fourth OWS candidate to enter Phase 3 clinical trials in the United States. Up to 60,000 volunteers will be enrolled in the trial at up to nearly 215 clinical research sites in the United States and internationally.
April
April 16: HHS made up to $483 million in support available for Moderna's candidate vaccine, which began Phase 1 trials on March 16 and received a fast-track designation from FDA. This agreement was expanded on July 26 to include an additional $472 million to support late-stage clinical development, including the expanded Phase 3 study of the company's mRNA vaccine, which began on July 27th.
May
May 21: HHS announced up to $1.2 billion in support for AstraZeneca's candidate vaccine, developed in conjunction with the University of Oxford. The agreement is to make available at least 300 million doses of the vaccine for the United States, with the first doses delivered as early as October 2020, if the product successfully receives FDA EUA or licensure. AstraZeneca's large-scale Phase 3 clinical trial began on August 31, 2020.
July
July 7: HHS announced $450 million in funds to support the large-scale manufacturing of Regeneron's COVID-19 investigational anti-viral antibody treatment, REGN-COV2. This agreement is the first of a number of OWS awards to support potential therapeutics all the way through to manufacturing. As part of the manufacturing demonstration project, doses of the medicine will be packaged and ready to ship immediately if clinical trials are successful and FDA grants EUA or licensure.
July 7: HHS announced $1.6 billion in funds to support the large-scale manufacturing of Novavax's vaccine candidate. By funding Novavax's manufacturing effort, the federal government will own the 100 million doses expected to result from the demonstration project.
July 22: HHS announced up to $1.95 billion in funds to Pfizer for the large-scale manufacturing and nationwide distribution of 100 million doses of their vaccine candidate. The federal government will own the 100 million doses of vaccine initially produced as a result of this agreement, and Pfizer will deliver the doses in the United States if the product successfully receives FDA EUA or licensure, as outlined in FDA guidance, after completing demonstration of safety and efficacy in a large Phase 3 clinical trial, which began July 27th.
July 31: HHS announced approximately $2 billion in funds to support the advanced development, including clinical trials and large scale manufacturing, of Sanofi and GlaxoSmithKline's (GSK) investigational adjuvanted vaccine. By funding the manufacturing effort, the federal government will own the approximately 100 million doses expected to result from the demonstration project. The adjuvanted vaccine doses could be used in clinical trials or, if the FDA authorizes use, as outlined in agency guidance, the doses would be distributed as part of a COVID-19 vaccination campaign.August
August 5: HHS announced approximately $1 billion in funds to support the large-scale manufacturing and delivery of Johnson & Johnson's (Janssen) investigational vaccine candidate. Under the terms of the agreement, the U.S. Government will own the resulting 100 million doses of vaccine, and will have the option to acquire more. The company's investigational vaccine relies on Janssen's recombinant adenovirus technology, AdVac, a technology used to develop and manufacture Janssen's Ebola vaccine with BARDA support; that vaccine received European Commission approval and was used in the Democratic Republic of the Congo (DRC) and Rwanda during the 2018-2020 Ebola outbreak that began in the DRC.
August 11: HHS announced up to $1.5 billion in funds to support the large-scale manufacturing and delivery of Moderna's investigational vaccine candidate. Under the terms of the agreement, the U.S. Government will own the resulting 100 million doses of vaccine, and will have the option to acquire more. The vaccine, called mRNA-1273, has been co-developed by Moderna and scientists from the National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health. NIAID has continued to support the vaccine's development including nonclinical studies and clinical trials. Additionally, BARDA has supported phase 2/3 clinical trials, vaccine manufacturing scale up and other development activities for this vaccine. The Phase 3 clinical trial, which began July 27, is the first government-funded Phase 3 clinical trial for a COVID-19 vaccine in the United States.
August 23: As part of the agency's efforts to combat COVID-19, the FDA issued an emergency use authorization (EUA) for investigational convalescent plasma. Based on available scientific evidence, the FDA determined convalescent plasma may be effective in lessening the severity or shortening the length of COVID-19 illness in hospitalized patients, and that the known and potential benefits of the product outweigh the known and potential risks. The EUA authorizes the distribution of convalescent plasma in the U.S. as well as its administration by health care providers, as appropriate, to treat suspected or confirmed cases of COVID-19. Learn more about EUAs.
October
October 9: HHS announced an agreement with AstraZeneca for late-stage development and large-scale manufacturing of the company's COVID-19 investigational product AZD7442, a cocktail of two monoclonal antibodies, that may help treat or prevent COVID-19. The goal of AstraZeneca's partnership with the U.S. Government is to develop a monoclonal antibody cocktail that can help prevent infection. An effective monoclonal antibody that can prevent COVID-19, particularly one that is long-lasting and delivered by intramuscular injection, may be of particular use in certain groups. This includes people who have compromised immune function, those who are over 80 years old, and people undergoing medical treatments that preclude them from receiving a COVID-19 vaccine.
October 28: HHS announced a $375 million agreement with Eli Lilly and Company to purchase the first doses of the company's COVID-19 investigational antibody therapeutic bamlanivimab, also known as LY-CoV555. Bamlanivimab currently is being evaluated in Phase 3 clinical trials funded by Eli Lilly, in addition to clinical trials as part of the ACTIV public-private partnership. The FDA is reviewing bamlanivimab as a possible treatment for COVID-19 in outpatients. Monoclonal antibodies, which mimic the human immune system, bind to certain proteins of a virus, reducing the ability of the virus to infect human cells.
November
November 10: HHS announced plans to allocate initial doses of Eli Lilly and Company's investigational monoclonal antibody therapeutic, bamlanivimab, which received emergency use authorization from the FDA on November 9, for the treatment of non-hospitalized patients with mild or moderate confirmed cases of COVID-19. A data-driven system will ensure continued fair and equitable distribution of these new products. Weekly allocations to state and territorial health departments will be proportionally based on confirmed COVID-19 cases in each state and territory over the previous seven days, based on data hospitals and state health departments enter into the HHS Protect data collection platform. To find out how much bamlanivimab has been allocated to specific states, territories, and jurisdictions, visit the allocation dashboard. This dashboard will be updated each distribution week until the FDA issues a revised EUA indicating the U.S. government involvement in the allocation and distribution process is no longer needed.
November 23: HHS announced plans to allocate initial doses of Regeneron's investigational monoclonal antibody therapeutic, casirivimab and imdevimab, which received emergency use authorization from the U.S. Food and Drug Administration on November 21, 2020, for treatment of non-hospitalized patients with mild or moderate confirmed cases of COVID-19 at high risk of hospitalization. In July, the federal government announced federal funding to support large-scale manufacturing of the therapeutic with approximately 300,000 doses of the medicine expected to result from the project. HHS will allocate these government-owned doses equitably on a weekly basis to state and territorial health departments which, in turn, will determine which healthcare facilities receive the infusion drug. To find out how much of Regeneron's therapeutic has been allocated to specific states, territories, and jurisdictions, visit the allocation dashboard. This dashboard will be updated each distribution week until the FDA issues a revised EUA indicating the U.S. government involvement in the allocation and distribution process is no longer needed.
December
December 11, 2020: HHS announced an agreement with Moderna to acquire an additional 100 million doses of their COVID-19 vaccine candidate, bringing the total doses of mRNA-1273 owned by the federal government to 200 million. Under the agreement, Moderna will leverage its U.S.-based manufacturing capacity to fill, finish and ship vials of mRNA-1273 as the bulk material is produced. The additional doses ordered today provide for continuous delivery through the end of June 2021. This federal funding brings the total provided to Moderna for this vaccine, including vaccine development, clinical trials and manufacturing, to $4.1 billion. The government also has the option to acquire up to an additional 300 million doses of the Moderna vaccine.
December 11, 2020: The FDA issued an Emergency Use Authorization (EUA) for Pfizer-BioNTech’s COVID-19 vaccine candidate, allowing the vaccine to be distributed in the U.S. The totality of the available data provides clear evidence that the known and potential benefits outweigh the known and potential risks, supporting the vaccine’s use in millions of people 16 years of age and older, including healthy individuals. In making this determination, the FDA can assure the public and medical community that it has conducted a thorough evaluation of the available safety, effectiveness and manufacturing quality information. The Pfizer-BioNTech COVID-19 vaccine contains messenger RNA (mRNA), which is genetic material. The vaccine contains a small piece of the SARS-CoV-2 virus’s mRNA that instructs cells in the body to make the virus’s distinctive “spike” protein. When a person receives this vaccine, their body produces copies of the spike protein, which does not cause disease, but triggers the immune system to learn to react defensively, producing an immune response against SARS-CoV-2. Read more about the EUA.
December 18, 2020: The FDA issued an Emergency Use Authorization (EUA) for Moderna's COVID-19 vaccine candidate, the second COVID-19 vaccine authorized for distribution in the U.S. The totality of the available data provides clear evidence that the known and potential benefits outweigh the known and potential risks, supporting the vaccine's use in millions of people 18 years of age and older. In making this determination, the FDA can assure the public and medical community that it has conducted a thorough evaluation of the available safety, effectiveness and manufacturing quality information. The Moderna COVID-19 Vaccine contains messenger RNA (mRNA), which is genetic material. The vaccine contains a small piece of the SARS-CoV-2 virus's mRNA that instructs cells in the body to make the virus's distinctive "spike" protein. After a person receives this vaccine, their body produces copies of the spike protein, which does not cause disease, but triggers the immune system to learn to react defensively, producing an immune response against SARS-CoV-2. Read more about the EUA.
As announced on May 15, the vaccine development plan is as follows, subject to change as work proceeds:
- Fourteen promising candidates have been chosen from the 100+ vaccine candidates currently in development—some of them already in clinical trials with U.S. government support.
- The 14 vaccine candidates are being narrowed down to about seven candidates, representing the most promising candidates from a range of technology options (nucleic acid, viral vector, protein subunit), which will go through further testing in early-stage clinical trials.
- Large-scale randomized trials for the demonstration of safety and efficacy will proceed for the most promising candidates.
Manufacturing
The federal government is making investments in the necessary manufacturing capacity at its own risk, giving firms confidence that they can invest aggressively in development and allowing faster distribution of an eventual vaccine. Manufacturing capacity for selected candidates will be advanced while they are still in development, rather than scaled up after approval or authorization. Manufacturing capacity developed will be used for whatever vaccine is eventually successful, if possible given the nature of the successful product, regardless of which firms have developed the capacity.
Select actions to support OWS manufacturing efforts so far include:
May
The May 21, April 16, and March 30 HHS agreements with AstraZeneca, Moderna, and Johnson & Johnson respectively include investments in manufacturing capabilities.
June
June 1: HHS announced a task order with Emergent BioSolutions to advance domestic manufacturing capabilities and capacity for a potential COVID-19 vaccine as well as therapeutics, worth approximately $628 million, using Emergent's BARDA-supported Center for Innovation in Advanced Department and Manufacturing.
July
July 27: HHS announced a task order with Texas A&M University and FUJIFILM to advance domestic manufacturing capabilities and capacity for a potential COVID-19 vaccine, worth approximately $265 million, using another BARDA-supported CIADM.
August
August 4: Grand River Aseptic Manufacturing Inc., (GRAM) Grand Rapids, Michigan, was awarded a $160 million firm-fixed-price contract for domestic aseptic fill and finish manufacturing capacity for critical vaccines and therapeutics in response to the COVID-19 pandemic.
October
October 13: HHS announced a $31 million agreement with Cytiva to expand the company's manufacturing capacity for products that are essential in producing COVID-19 vaccines, such as liquid and dry powder cell culture media, cell culture buffers, mixer bags, and XDR bioreactors. Cytiva is a major manufacturer of pharmaceutical consumables and hardware products and the primary supplier to many of the companies currently working with the U.S. government to develop COVID-19 vaccines. This capacity expansion will help Cytiva respond to the demand for COVID-19 vaccine consumables and hardware products without impacting on current manufacturing output.
Distribution
OWS and our private partners are developing a plan for delivering a safe and effective product to Americans as quickly and reliably as possible. Experts from HHS are leading vaccine development, while experts from DoD are partnering with the CDC and other parts of HHS to coordinate supply, production, and distribution of vaccines.
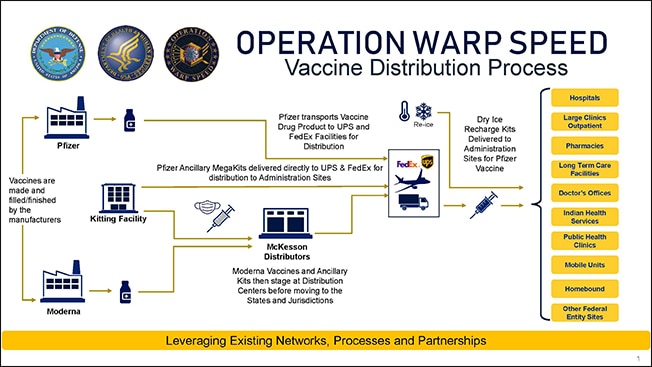
Download the Vaccine Distribution Process*
Select actions to support OWS distribution efforts include:
May
May 12: DoD and HHS announced a $138 million contract with ApiJect for more than 100 million prefilled syringes for distribution across the United States by year-end 2020, as well as the development of manufacturing capacity for the ultimate production goal of over 500 million prefilled syringes in 2021.
June
June 9: HHS and DoD announced a joint effort to increase domestic manufacturing capacity for vials that may be needed for vaccines and treatments
June 11: HHS announced $204 million in funds to Corning to expand the domestic manufacturing capacity to produce approximately 164 million Valor Glass vials per year if needed. Valor Glass provides chemical durability to minimize particulate contamination. The specialized glass allows for rapid filling and capping methods that can increase manufacturing throughput by as much as 50 percent compared with conventional filling lines, which in turn can reduce the overall manufacturing time for vaccines and therapies.
June 11: HHS announced $143 million to SiO2 Materials Science to ramp up capacity to produce the company's glass-coated plastic container, which can be used for drugs and vaccines. The new lines provide the capacity to produce an additional 120 million vials per year if needed.
August
August 14: HHS and DoD announced that McKesson Corporation will be a central distributor of future COVID-19 vaccines and related supplies needed to administer the pandemic vaccinations. The CDC is executing an existing contract option with McKesson to support vaccine distribution. The company also distributed the H1N1 vaccine during the H1N1 pandemic in 2009-2010. The current contract with McKesson, awarded as part of a competitive bidding process in 2016, includes an option for the distribution of vaccines in the event of a pandemic. Detailed planning is underway to ensure rapid distribution as soon as the FDA authorizes one or more vaccines. Once these decisions are made, McKesson will work under CDC's guidance to ship COVID-19 vaccines to administration sites.
September
September 16: HHS and DoD released two documents outlining the Trump Administration's detailed strategy to deliver safe and effective COVID-19 vaccine doses to the American people as quickly and reliably as possible. The documents, developed by HHS in coordination with DoD and the Centers for Disease Control and Prevention (CDC), provide a strategic distribution overview along with an interim playbook for state, tribal, territorial, and local public health programs and their partners on how to plan and operationalize a vaccination response to COVID-19 within their respective jurisdictions.
October
October 16: HHS and DoD announced agreements with CVS and Walgreens to provide and administer COVID-19 vaccines to residents of long-term care facilities (LTCF) nationwide with no out-of-pocket costs. Protecting especially vulnerable Americans has been a critical part of the Trump Administration's work to combat COVID-19, and LTCF residents may be part of the prioritized groups for initial COVID-19 vaccination efforts until there are enough doses available for every American who wishes to be vaccinated. The Pharmacy Partnership for Long-Term Care Program provides complete management of the COVID-19 vaccination process. This means LTCF residents and staff across the country will be able to safely and efficiently get vaccinated once vaccines are available and recommended for them, if they have not been previously vaccinated. It will also minimize the burden on LTCF sites and jurisdictional health departments of vaccine handling, administration, and fulfilling reporting requirements.
November
November 12: HHS and DoD announced partnerships with large chain pharmacies and networks that represent independent pharmacies and regional chains. Through the partnership with pharmacy chains, this program covers approximately 60 percent of pharmacies throughout the 50 states, the District of Columbia, Puerto Rico, and the U.S. Virgin Islands. Through the partnerships with network administrators, independent pharmacies and regional chains will also be part of the federal pharmacy program, further increasing access to vaccine across the country—particularly in traditionally underserved areas.
Who's leading Operation Warp Speed?
HHS Secretary Alex Azar and Acting Defense Secretary Christopher Miller oversee OWS, with Dr. Moncef Slaoui designated as chief advisor and General Gustave F. Perna confirmed as the chief operating officer. To allow these OWS leaders to focus on operational work, in the near future the program will be announcing separate points of contact, with deep expertise and involvement in the program, for communication with Congress and the public.
What are you doing to make these products affordable for Americans?
The Administration is committed to providing free or low-cost COVID-19 countermeasures to the American people as fast as possible. Any vaccine or therapeutic doses purchased with US taxpayer dollars will be given to the American people at no cost.
How is Operation Warp Speed being funded?
Congress has directed almost $10 billion to this effort through supplemental funding, including the CARES Act. Congress has also appropriated other flexible funding. The almost $10 billion specifically directed includes more than $6.5 billion designated for countermeasure development through BARDA and $3 billion for NIH research.
*This content is in the process of Section 508 review. If you need immediate assistance accessing this content, please submit a request to digital@hhs.gov.