Approved July 20, 2022
I. Introduction
A threshold question in determining whether the Common Rule applies to a given research activity is determining whether a Federal Agency that has adopted the Common Rule is providing “support” for the research. In the May 2021 Charge to SACRHP entitled “Reimagining Engagement” the third section of the charge regarding support states:
Embedded into the application of the HHS regulations is the threshold concept of jurisdiction, HHS conducted or supported research. While the interpretation of HHS conducted research is straightforward, the meaning of HHS support may be less direct and has often required interpretation. The FWA Terms of Assurance state only that, “For the purposes of the FWA, federally supported means the U.S. Government is providing any funding or other support,” and OHRP has historically interpreted support to include, funding, the provision of identifiable private information, or other tangible support such as the provision of a study drug. What should be included in the concept of HHS support, besides funding of the research grant?
The primary purpose of this SACHRP recommendation is to provide a clear definition of “support” that will provide the regulated community, including HHS, with consistency in determining when research is supported by HHS, which in turn should ease administrative burden. The easing of administrative burden must be appropriately balanced with providing appropriate protection to research subjects through application of the Common Rule. This recommendation does not address agencies other than HHS.
While the main focus of this recommendation is research conducted in the United States, one area of focus is international research. In this setting, the goal to not inappropriately overreach into other countries, particularly when US based researchers are collaborators in international research. International collaboration could be stifled if an HHS-supported investigator is involved and thus the entire study falls under the Common Rule requirements.
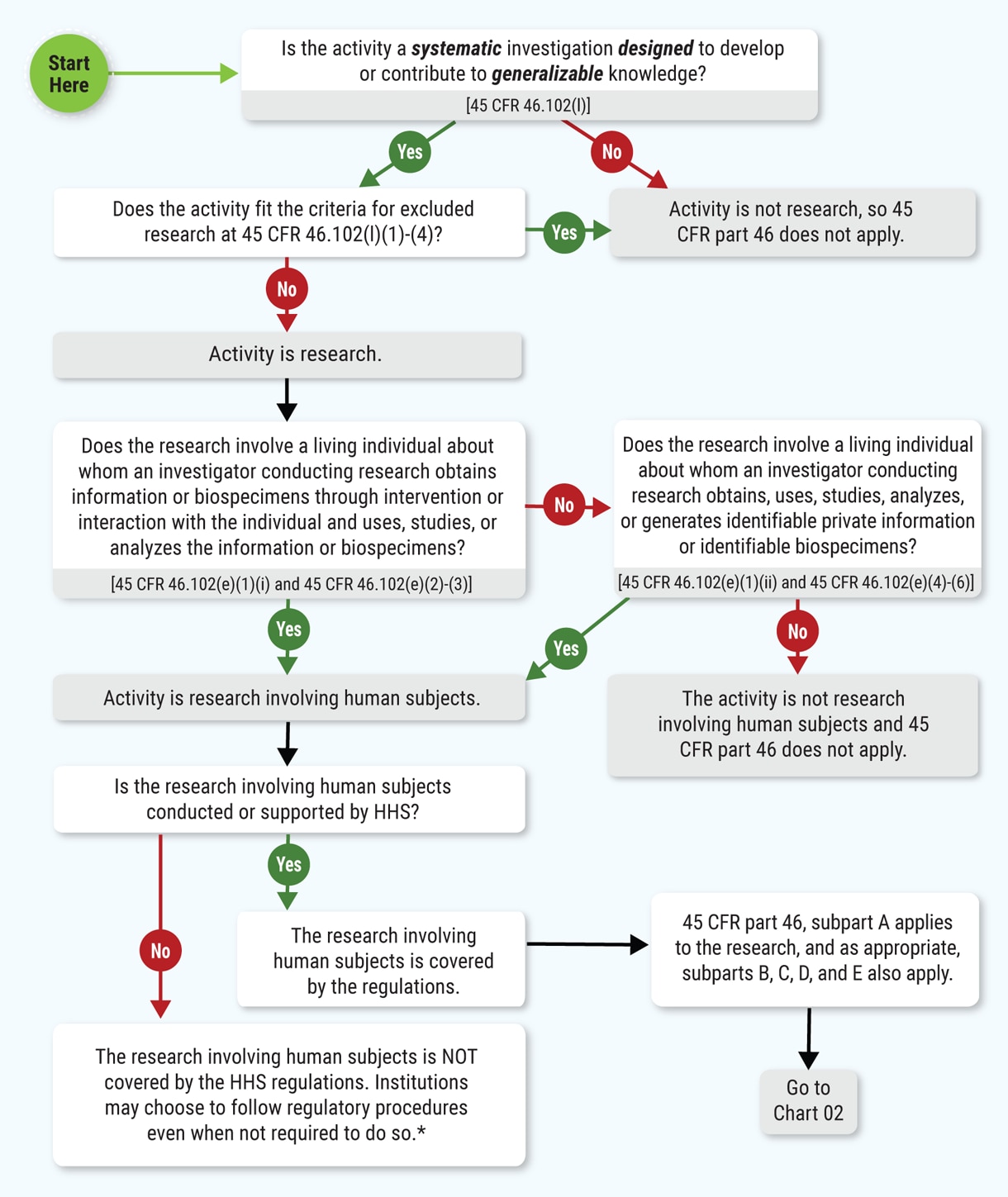
II. OHPR Decision Chart 01
For framing purposes, here is the OHRP Decision Chart 01, “Is an Activity Human Subjects Research Eligible for Exemption under 45 CFR 46.104(d)?” Note that the issue of being “conducted or supported by HHS” is located about two-thirds of the way down.
Chart 01: Is an Activity Human Subjects Research Covered by 45 CFR Part 46?
III. OHRP List of Scenarios that Would Constitute “HHS-Supported” and “Not HHS-Supported”
Currently, OHRP has presented SACHRP with the following list of scenarios that constitute human subjects research that is supported and not supported:
HHS-Supported:
- Funding for specific human subjects research studies (including direct awardee and sub-awardees)
- Providing non-financial, tangible goods for a specific human subjects research study (e.g., supplies, products, or drugs)
- Providing HHS-held identifiable private information for research (e.g., beneficiary data held by CMS)
- HHS funds or conducts laboratory or data analysis that will be used in a way that impacts the involvement of research subjects (e.g., to determine subject eligibility, or study group assignment)
Not HHS-Supported
- Funding for general research infrastructure
- Block grants (e.g. to state organizations)
- Involvement by HHS employees in developing human subject research protocols
- Involvement of HHS employees in monitoring research
- Conduct of research by HHS employees or HHS-funded researchers in research that is not otherwise supported by HHS
- When compliance with the HHS human subject protection regulations is a condition of eligibility for an HHS program (e.g., CMS’s Coverage with Evidence Development Program requires compliance with 45 CFR Part 46 as a condition of eligibility).
The purpose of this recommendation is to work with this initial list to come up with a consistent approach to determining whether research is HHS-Supported or not. First, we analyze each of the OHRP scenarios individually, and second, we propose a consolidated rule for determining when a research project is supported by HHS.
Analysis of the OHRP List of Scenarios that Would Constitute “HHS-Supported”
- HHS-Supported: Funding for specific human subjects research studies (including direct awardee and sub-awardees).
Recommendation: SACHRP agrees with this analysis.
Rationale for considering this to be “support”: The human subjects research is funded by HHS through the award of a grant for the purpose of conducting the research, and therefore the plain reading of the regulatory language is that this is HHS-supported research.
- HHS-Supported: Providing non-financial, tangible goods for a specific human subjects research study (e.g., supplies, products, or drugs)
Recommendation: SACHRP recommends that this scenario be kept, but with some clarifying information.
First, provide a definition of “tangible” or use a different word or phrase to clarify the meaning. In one dictionary, there are four definitions of tangible:
- capable of being touched; discernible by the touch; material or substantial.
- real or actual, rather than imaginary or visionary: the tangible benefits of sunshine.
- definite; not vague or elusive: no tangible grounds for suspicion.
- (of an asset) having actual physical existence, as real estate or chattels, and therefore capable of being assigned a value in monetary terms.
For instance, the scenario should clarify whether the tangible goods include only concrete items that can be assigned value, such as real estate and chattel, or whether they can also include goods that are tangible in the sense of having financial value, such as intellectual property. If intellectual property and other goods that are tangible in the sense of having financial value, it would also be helpful to clarify what type of documentation of such value is expected or required.
Second, clarify that this scenario does not encompass the use of items that were acquired during the conduct of previous HHS funded research, such as the use of a centrifuge that was purchased with a prior grant for a specific research study. This is established precedent in HHS funding processes.
Third, clarify whether this scenario encompasses tangible goods acquired with separate infrastructure grants. [note – this is also addressed below in not research scenario 1.]
Fourth, clarify that this scenario does not include publicly available tangible goods that were developed with HHS funding. For example, if NIH has developed a toolbox for assessing neurological function, and it is publicly available, it does not constitute HHS “support” if the toolbox is used in a research study.
Finally, clarify that there must be documentation that HHS intended to provide the tangible goods to this specific human subjects research study, such as a material transfer agreement, data use agreement, contract for access to data, etc.
- HHS-Supported: Providing HHS-held identifiable private information for research (e.g., beneficiary data held by CMS)
Recommendation: SACHRP supports this scenario as presented.
Rationale for considering this to be “support:” HHS is providing data that make it possible to do the research, and thus qualifies as support.
- HHS-Supported: HHS funds or conducts laboratory or data analysis that will be used in a way that impacts the involvement of research subjects (e.g., to determine subject eligibility, or study group assignment)
Recommendations: SACHRP recommends that this scenario be kept, but it should be narrowly interpreted so that the application is predictable.
First, this scenario should require that there be some documented evidence of HHS intent to support this specific research study, such as a contract for services.
Second, the scenario is currently confusing because it includes the phrase, “that will be used in a way that impacts the involvement of research subjects (e.g., to determine subject eligibility, or study group assignment).” For clarity, the scenario should be shortened to just the first phrase, in other words, “HHS funds or conducts laboratory or data analysis.” This removes the confusing requirement to determine if the HHS involvement “impacts the involvement of research subjects.”
Third, the scenario should clarify that it applies to both the involvement of an HHS employee or an HHS department or unit and is not limited to the involvement of individual HHS employees. Alternatively, that could be a separate scenario, but the same basic criteria should apply.
Fourth, the scenario should be equally applicable to domestic and international sites.
Finally, HHS should consider whether there should be a separate scenario that focuses on “HHS conduct” of research as opposed to “HHS support.” This scenario currently addresses both support and conduct, even though it is theoretically limited to support only.
Analysis of the OHRP List of Scenarios that Would Constitute “Not HHS-Supported”
- Not HHS-Supported: Funding for general research infrastructure
Recommendation: Research infrastructure grants are provided to institutions to enhance their ability to support research activities. SACHRP did not reach consensus on this issue. Therefore, SACHRP is recommending that the HHS and other agencies should consider the following arguments for and against in making their policy decision and also solicit feedback from research sites and other stakeholders regarding the potential operational impacts if research that is conducted with assistance or support from HHS funding is considered HHS Supported research.SACHRP also notes that there are several types of funding mechanisms that could be considered “funding for general research infrastructure.” The agencies should also address whether there might be distinctions in determining these various types of funding mechanisms should be considered to constitute support. Examples of funding mechanisms that should be considered are available at https://orip.nih.gov/funding/table-summary-grants-programs and https://grants.nih.gov/Grants/funding/funding_program.htm#PSeries.
The rationale for considering funding for general research infrastructure to be “support” is that it provides protection to research subjects by ensuring protections provided by the Common Rule in studies that are in fact in some significant or material way supported by HHS funds. Infrastructure support for such activities as hemophilia centers, cancer centers, and oncology groups provides core funding for administration, protocol writing, monitoring and even research team salaries. The goal of the Common Rule is to ensure that research conducted with federal funds is consistent with applicable ethical standards. The research included, however, should only be research that is conducted during the funding support period, and after funding has lapsed, research conducted within the institution, even if using equipment or supplies purchased during the funding period, would not be regarded as HHS-Supported.
There are also several arguments against considering this to be “support” including the following:
If this type of funding were considered to be “support” it would create administrative burden. For instance, HHS and institutions would need to come up with criteria for determining the nexus between the infrastructure grants and research studies that do not otherwise have federal support. For instance, is use of the grant to provide research staff salaries sufficient to create the nexus for the non-federally supported studies that they are working on? Is the use of piece of equipment purchased with an infrastructure grant in a non-federally supported study sufficient to create such a nexus?
Second, this approach is inconsistent with the frame of the Common Rule, which is designed for application to individual research studies.
Third, it would be difficult to interpret in certain situations. For instance, if the funding is only provided to one laboratory or department within an institution, does that mean that only that laboratory or department is “supported,” or is the entire institution “supported?”
Fourth, with the regulatory and NIH policy support for single IRB review of multi-site research, this could create a situation where some sites in a given multi-site protocol are HHS-supported while others are not. Thus, certain Common Rule requirements would apply to some sites in the research, such as reporting unanticipated problems and serious or continuing non-compliance to OHRP, ensuring consent forms have certain requirements such as a key information summary as required under 45 CFR 46.116(a)(5), and that the consent forms be publicly posted as required under 45 CFR 46.116(h). These are not required for studies under FDA jurisdiction, for instance, and would lead to differences among sites.
- Not HHS-Supported: Block grants (e.g., to state organizations)
Recommendation: SACHRP agrees with this scenario. Block grants are given by the federal government to individual states and local governments to support broad purpose programs, distinct from infrastructure grants.The rationale for considering funding for general research infrastructure to be “support” is that it provides protection to research subjects by ensuring protections provided by the Common Rule.
However, there are also several arguments against considering this to be “support:”
First, similarly to the scenario regarding infrastructure grants directly above, it would lead to some research that would not otherwise be supported to be supported. Institutions will need to establish the nexus and determine which studies are thus supported, which creates administrative burden. It may be difficult to trace the use of the block grant funding to a particular research project involving human subjects because there is no established nexus for linking the block grant funding to a given research project.
Second, this approach is inconsistent with the frame of the Common Rule, which is designed for application to individual research studies.
Third, due to the regulatory and NIH policy support for single IRB review of multi-site research, this could create a situation where some sites in a given multi-site protocol are federally funded while others are not. This would create complications in determining reporting requirements for issues such as unanticipated problems and serious or continuing non-compliance. This in turn could affect decisions one which sites to choose for multi-site research that is not federally funded, such as FDA regulated research that does not have federal funding.
- Not HHS-Supported: Involvement by HHS employees in developing human subject research protocols
Recommendation: SACHRP agrees with this scenario in principle. However, it needs some clarification.
First, this appears to extend “support” to development of research activities, which is not necessarily the same as the conduct of research. If the development of research is considered to be a subset of the conduct of research, that should be clarified.
Second, if development is a subset of supporting or conducting research, then we recommend that this scenario be modified to be congruent with Scenario four of HHS supported activities as addressed above, or even dropped and be included in Scenario four of HHS supported activities above.
Third, if development is considered a subset of supporting or conducting research, and this scenario is kept, it should include criteria that can be applied in defining what constitutes development activities.
- Not HHS-Supported: Involvement of HHS employees in monitoring research
Recommendation: SACHRP recommends that this scenario be modified to be congruent with Scenario four of HHS supported activities as addressed above.
Also, it would be helpful to tie “monitoring research” to a regulatory requirement, to clarify whether it is the definition from FDA regulations 21 CFR 312 and 21 CFR 812, or a different regulation.
Finally, HHS may wish to consider whether there should be a separate analysis of this scenario as the “conduct” of research, rather than “support” of research.
- Not HHS-Supported: Conduct of research by HHS employees or HHS-funded researchers in research that is not otherwise supported by HHS
Recommendation: SACHRP recommends that this scenario be modified to be congruent with Scenario four of HHS supported activities as addressed above. However, it should be noted that the details of this scenario are unclear.
- Not HHS-Supported: When compliance with the HHS human subject protection regulations is a condition of eligibility for an HHS program (e.g., CMS’s Coverage with Evidence Development Program requires compliance with 45 CFR Part 46 as a condition of eligibility).
Recommendation: SACHRP agrees with this scenario.
IV. General Rule
In this section we attempt to consolidate the scenarios above into a general rule (or definition) that determines when a research project is supported by HHS. Further work on this proposed rule would be necessary.
The most difficult part of this consolidation is harmonizing those scenarios that involve participation in the conduct of research by HHS employees or departments, specifically when they are:
- providing services such as laboratory or data analysis,
- developing human subject research protocols,
- conducting research that is not otherwise supported by HHS, and,
- providing monitoring services
Proposed Rule: HHS “support” is defined as:
- the provision of funding or items of value (such as equipment or labor) for a research project, and,
- for which there is documented evidence of the support specific to the research study such as a grant, a service contract, letter of support, or a material transfer agreement.
Based on this proposed rule, these would be examples of factors that lead to specific research projects being HHS supported:
- Documented HHS funding for the specific research project
- Documented provision of equipment for the specific research project
- Documented provision of HHS held private identifiable data for the specific research project
Based on this proposed rule, this will lead to specific research projects not being HHS supported:
- Conduct or support that does not involve documentation such as a grant or services agreement
V. Conclusion
HHS should provide clear guidance on the meaning of support, so that institutions and other parties involved in the conduct of research can accurately determine when a given research project has HHS support or not.

