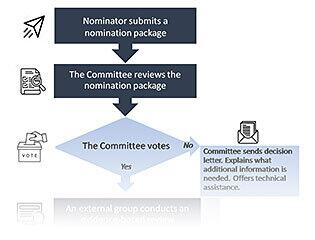
Adding a condition to the RUSP is a multistep process which begins with compiling a nomination package and may or may not result in the Committee recommending the nominated condition for inclusion on the RUSP. This flowchart depicts the nomination, evidence-based review and decision-making process and includes points at which the Committee votes on whether or not to move a nominated condition forward to the next step.
The ACHDNC Nomination, Review, and Decision-Making Process
 Download the flowchart graphic (PDF - 883 KB)
Download the flowchart graphic (PDF - 883 KB)
Nominator Submits a Nomination Package
The first step of adding a condition to the RUSP is to nominate the condition to the Committee. Anyone can nominate a condition by completing a nomination package. Preparing this package requires a lot of detailed data. To start the process, a team of experts and stakeholders that may include researchers, doctors, advocacy group members, and others work together to complete a nomination package.
A nomination package has 5 parts:
- Cover Letter
This letter introduces the condition and team. - Letters of Support
Members of the team and other individuals can write letters to support the nomination. - Conflict of Interest Form
The Conflict of Interest Disclosure Form* (PDF - 63 KB) clearly lays out any potential conflicts of interest, including financial ones. All members of the team fill out this form. - Nomination Form
The Nomination Form** (PDF - 2.57 MB) is the main part of the nomination package. It answers specific questions about screening and treatment for the nominated condition using data and evidence. - Supporting References and Data
This part lists scientific and clinical reports that support answers on the Nomination Form.
Preparing a nomination package is a big job that takes a lot of time and many people. To learn more, visit the Nominate a Condition and Nominate a Condition FAQs pages.
When the nomination package is ready, the nominator submits it to the Committee’s Designated Federal Official at achdnc@hrsa.gov. HRSA officials review the package to make sure it is complete, then forward it to the Committee’s Nomination and Prioritization (N&P) Workgroup for review.
*A potential or actual conflict of interest exists when commitments and obligations are likely to be compromised by the nominator(s)'s other material interests or relationships (especially economic), particularly if those interests or commitments are not disclosed.
**This form uses dynamic Adobe Acrobat features that may not be compatible with assistive technology. Individuals using assistive technology can use the standard version of the nomination form (PDF - 247 KB) instead.
Preparing a Nomination Package
Nominators and their teams do not need to contact HRSA when preparing a nomination package. However, HRSA officials can help nominators by making sure a nomination package has all the needed parts.
Preparing a nomination package entails compiling a large amount of information. Here are a few common oversights that can delay the review of the package, so avoiding them is important:
- Not listing enough reports to support the answers in the Nomination Form section
- Not having the right expertise on the nomination team needed to address the questions in the package
- Not including all required documents (e.g., conflict of interest forms)
Avoiding these mistakes can shorten the time it takes to review a nomination package.
Committee Reviews the Nomination Package
The Nomination and Prioritization (N&P) Workgroup reviews the nomination package to create a summary for the Committee’s assessment. The Committee Chair selects members for the N&P Workgroup.
During its review, the N&P Workgroup carefully considers key questions about the condition, screening process, and treatment. It identifies anything missing from the package or needed to complete the review and, if necessary, asks the nominator for more information.
After careful review and discussion, the N&P Workgroup presents its summary to the Committee. To learn how the Committee assesses the summary, visit the Committee Voting section.
Key Questions Related to Nominated Conditions
The Committee evaluates the nomination package and other sources of evidence to better understand whether newborn screening could improve health outcomes. They follow key questions to guide their assessment of issues related to the condition, screening, and treatment, such as:
- Is the condition serious?
- What do we know about the condition?
- How accurate is screening at finding newborns with the condition?
- Are treatment(s) available for the condition?
- Are there data showing how well screening works in the population?
The specific questions that the Committee use to review the nomination package are listed on the Key Questions Considered by the Committee page.
Pilot Studies
The Committee also reviews pilot study data submitted in the nomination package. At the time of nomination existing pilot study data should be able to:
- Describe how well the condition’s screening process works, including its accuracy.
- Describe the benefit of detecting the condition with screening versus in the clinic after symptoms appear.
- Find at least one case of the condition through screening using methods relevant to the U.S.
For more details about this topic, view the Pilot Study Workgroup’s 2016 presentation, summary of the full Pilot Study Workgroup report, or the full Report and Recommendations of the Pilot Study Workgroup.
Committee Votes on Whether or Not to Send a Nomination to Evidence-Based Review
After the N&P Workgroup presents its summary, the Committee discusses the condition. It talks about the key questions, hears any public comments, and then votes on whether to move the condition forward for evidence-based review.
If the Committee votes no, it sends a decision letter to the nominator explaining the specific reasons for the vote and describing what new information would be needed to reconsider the nomination at a later time.
External Group Conducts an Evidence-Based Review
If the Committee accepts a nomination, the condition moves forward for an evidence-based review. An evidence-based review is a thorough review of data on the impact of newborn screening for the condition.
Evidence-Based Review Group
An external group called the Evidence-Based Review Group (ERG) collects data, creates a final report, and presents the report to the Committee.
The ERG is an external, independent team that includes pediatricians, clinical specialists, newborn screening researchers, health economists, people from state newborn screening labs, technical experts, and others.
By rule, members of the ERG cannot be members of the Committee at the same time. This rule separates the people involved in each step of a condition’s review and prevents any conflicts or bias.
Guiding Questions Used by the ERG
The following table outlines the three guiding questions in an evidence-based review, along with the content and methods used to answer each. You can also view evidence-based review reports for previously nominated conditions.
| Guiding Question | Main Content | Methods |
|---|---|---|
| What are the clinical effects of early detection and treatment for the condition? | Benefits for newborns and families Answers to this question consider reported outcomes (both benefits and harms) of early detection and treatment for newborns, caregivers, and families. |
Systematic evidence review This method involves review of published and unpublished reports. |
| How would newborn screening for the condition affect the population? | Benefit for the U.S. population Answers to this question predict how many newborns with the condition screening would find in the U.S. and how many of those newborns would have better outcomes because of screening. |
Decision analysis modeling This method uses data to predict how adding screening would affect the U.S. population of newborns. |
| How would adding newborn screening services for the condition affect the public health system? | Public health system readiness Answers to this question assess:
|
Public health system impact assessment This method involves gathering information from state newborn screening programs about adding screening for the condition. |
Topic Areas in an Evidence-Based Review
The ERG explores eight topic areas during an evidence-based review. The specific questions to address within each topic area are adapted for each condition under review. Examples of the types of questions which might be addressed within each topic area can be found here. The topic areas are:
- Epidemiology with Clinical Detection and Usual Care
This topic reviews data about how many people have the condition; when symptoms appear; and the condition’s typical course without newborn screening. - Screening
This topic reviews the screening process. It covers details of the screening process, how well it works, and whether screening predicts the condition’s form or severity. - Short-Term Follow-Up and Diagnosis
This topic reviews data on short-term follow-up of newborns at high risk for the condition. It covers whether testing to confirm a diagnosis is available, accessible, and feasible. - Benefits and Harms of Screening and Diagnosis (Not Related to Treatment)
This topic reviews benefits and harms, not related to treatment, that could result from newborn screening and early diagnosis. Many benefits and harms affect both the newborn and family. - Treatment and Long-Term Follow-Up Care
This topic reviews current treatment practices and guidelines. It covers treatment types, details, and duration and whether treatment changes based on age or symptoms. - Outcomes of Early Detection and Treatment
- Primary Health Outcomes - Reviews the condition’s primary health outcomes (e.g., survival, age of mortality). It may also cover how other measures relate to these outcomes.
- Secondary Health Outcomes - Reviews other health outcomes related to the condition.
- Intermediate Outcome Measures - Considers whether and how much treatment and long-term follow-up affect other measures.
- Benefits and Harms of Treatment and Long-Term Follow-Up
This topic reviews the benefits and harms of treatment and long-term follow-up care for newborns found with versus without newborn screening. - Public Health Impact
This topic reviews the impact of adding newborn screening for the condition on population health and the public health system.- Population Health - Covers the impact of newborn screening on the population by predicting how many babies would be found and treated with versus without newborn screening.
- Public Health System Impact - Covers the resources, feasibility, and readiness of state newborn screening programs to begin screening for the condition and the costs to start screening.
The ERG updates the Committee as it prepares its report. When the report is ready, the ERG presents it to the Committee. See examples of past External Evidence Review Reports.
The Committee Considers the Evidence
After the ERG presents its report, the Committee reviews and discusses the report. It focuses on the overall benefit and harms of screening for the nominated condition. Following the Committee discussion, members use a rating system to assess evidence on a) net benefit to the newborn and b) feasibility and readiness of state programs to expand screening for the condition. The Committee gives a separate rating for each of these topics (Table 2 and 3):
- The net benefit of newborn screening for the condition considers clinical and health outcomes of early detection and treatment.
- The feasibility and readiness of the public health system to expand newborn screening.
| Rating | Description |
|---|---|
| A | High certainty that screening for the targeted condition would lead to a significant net benefit |
| B | Moderate certainty that screening for the targeted condition would lead to a significant benefit |
| C | High or moderate certainty that screening for the targeted condition would lead to a small to zero net benefit |
| D | High or moderate certainty that screening for the targeted condition would lead to a negative net benefit |
| L | Low certainty regarding the net benefit of screening |
Note: Moderate certainty indicates that the Advisory Committee believes that further research could change the magnitude or direction of findings within any of the key questions.
| Rating | Description |
|---|---|
| 1 | Screening for the targeted condition has high or moderate feasibility and most newborn screening programs are ready for comprehensive screening |
| 2 | Screening for the targeted condition has high or moderate feasibility and most newborn screening programs have developmental readiness for comprehensive screening |
| 3 | Screening for the targeted condition has high or moderate feasibility and most newborn screening programs are unprepared for comprehensive screening |
| 4 | Screening for the targeted condition has low feasibility |
Note: High or moderate feasibility is based on the Advisory Committee’s determination that there is an established and available screening test that can be adopted, a clear approach to diagnostic confirmation, and a treatment plan that is acceptable to clinicians and affected individuals and their families, and plans for long-term follow up can be established.
The Committee rates screening benefits based on two main factors. The first factor is the size of the benefits, which reflects how much treatment or screening helps newborns with the condition. The second factor is the Committee’s confidence in any screening benefits, which reflects whether the Committee believes reports about them are valid and could be repeated.
The Committee rates feasibility and program readiness based on two main factors. The first factor of feasibility is rated as high/moderate or low. A rating of high/moderate means that a screening process, a clear approach to confirm diagnosis, an acceptable treatment plan, and a long-term follow-up strategy all exist for the condition.
The second factor is the Committee’s rating of state newborn screening programs’ readiness to begin screening as ready, developmental readiness, or unprepared. A rating of ‘ready’ indicates that a state has:
- The resources needed for screening, confirming a diagnosis, and long-term follow-up.
- The equipment, data systems, and expertise needed for screening.
- Access to specialty care and treatments.
- Systems for data collection.
- Approval for screening.
After the Committee rates the evidence, it uses a decision matrix to organize its ratings. Learn more in this report  and on the Committee Approach to Evaluating the Condition Review Report (Decision Matrix) page.
and on the Committee Approach to Evaluating the Condition Review Report (Decision Matrix) page.
Committee Votes on Whether or Not to Recommend Addition to the RUSP
After considering the evidence from the ERG report, the Committee votes on whether to recommend adding the condition to the RUSP. The 2014 Reauthorization of the Newborn Screening Saves Lives Act puts a time limit on this step. The act requires the Committee to vote on recommending a condition within 9 months of accepting its nomination.
If the Committee votes no, then it does not recommend adding the condition to the RUSP. The Committee sends a decision letter to the nominator explaining the reasons for the vote and describing what new information would be needed to reconsider the condition at a later time.
The Committee Makes a Recommendation
If the Committee votes yes, it recommends adding the nominated condition to the RUSP. The Committee Chair writes a letter to the HHS Secretary explaining the Committee’s recommendation and reasoning. Read previous recommendation letters from the Committee.
The HHS Secretary receives the Committee’s letter, then reviews its recommendation with officials from other HHS agencies. Based on the evidence and Committee’s recommendation, the Secretary decides whether to add the condition to the RUSP.
Read past Secretary’s responses for previously nominated conditions.